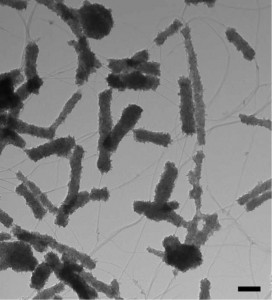
Transmission electron microscopy image of core–shell hybrid titania-SWCNT material. The “puffy” morphology of titania originates in nucleation of titania on single wall carbon nanotube defect sites. Credit: Star; University of Pittsburgh.
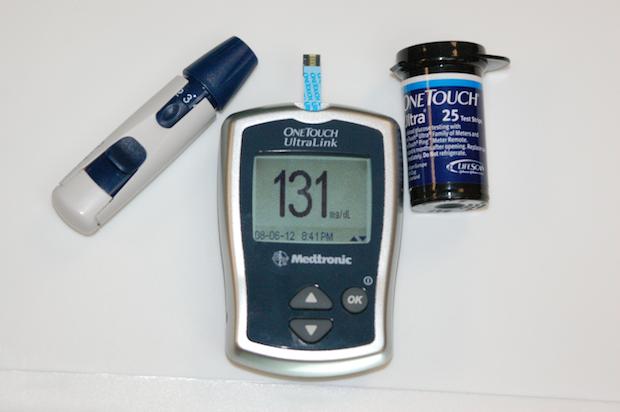
Image: Advances in nanomaterials may allow diabetes patients to toss glucose monitoring equipment in the dumpster in favor of a handheld breathalyzer. Credit: Rawson; TPCC.
One of the oldest diagnostic tools used by medical professionals are their noses. We know, for example, that Hippocrates of Cos smelled breath as part of his diagnostic process.
“The human nose is very sophisticated,” says University of Pittsburgh associate professor of chemistry Alexander Star, in a phone interview. “And, it is connected to a very sophisticated computer—the brain.”
Of the thousand-or-so compounds that comprise human breath, about 30 have been identified, and most of those are potential indicators—or biomarkers—of disease, according to a September 2012 ACerS Buletin article (pdf) by Perena Gouma. Example biomarker compounds are nitrogen oxides for lung inflammation, isoprene for blood cholesterol, ammonia for renal disease, and acetone for diabetes.
While the human nose is very good at detecting the presence of certain compounds, it cannot quantify levels, nor can it detect low threshold amounts (and pity the poor allergy-afflicted physician).
Gouma and her team at the State University of New York, Stony Brook, have made significant advances in electrospinning metal oxide nanowires that detect specific disease biomarkers in exhaled breath. (The ACerS Bulletin article highlights work on metastable molybdenum oxide and tungsten polymorphs.) A new paper published in the Journal of the American Chemical Society (fee or subscription required) demonstrates another approach to developing a material that detects very low levels of acetone and could revolutionize the diagnosis and management of diabetes.
The group, led by Star, started with single wall carbon nanotubes functionalized with oxygen. Using a sol gel process, they deposited titanium dioxide to make core–shell hybrid nanostructures. In a phone interview, Star explained that the oxygen on the SWCNT surfaces provides an “anchoring” or nucleation site for titania to deposit. The deposited titania was mostly amorphous, but after calcining at 500°C in argon for 30 minutes, Raman spectroscopy showed evidence of anatase.
The SWCNT are not simply substrates, though. “Single wall carbon nanotubes are basically all surface atoms and are sensitive to local changes,” Star explains. “The trouble is that acetone does not change the local environment much by itself.”
The intimate contact between the titania and SWCNTs that the core–shell structure affords provides a mechanism for building a chemiresistor that is sensitive to very low acetone levels—as low as 2–20 ppm, according to the paper. Based on their simulations, the researchers think detecting concentrations levels as low as 0.4 ppm is possible.
The group used UV light to sensitize the titania to acetone. (The photoexcitation properties of titania are well known and also used in photovoltaic and photocatalytic applications). According to Star, the titania gives up electrons to the SWCNT on illumination. The resulting holes allow acetone vapor to transfer charge to the titania. As that happens, a detectable current sets up in the SWCNTs.
The paper reports on a systematic study of hybrid structures with different morphologies and interfaces. The scientists also used density functional theory calculations to model the charge transfer mechanisms that produce the ultrahigh sensitivity of the composite to acetone. The hybrid nanowire-based sensor is capable of “fast, linear, and reversible detection of acetone vapors with concentrations in a few parts per million range.” The group is working on developing a device for clinical tests.
Star says the work on detecting acetone grew out of the group’s previous work studying SWCNT functionalized with polymers to detect NOx for asthma and SWCNTs functionalized with gold to detect hydrogen sulfide, the culprit responsible for halitosis. As Star says, “You cannot smell your own bad breath!”
The bad breath sensor research was sponsored by Colgate, the dental hygiene products manufacturer. Maybe there is a collaboration opportunity with the social science research community?
The National Energy Technology Laboratory supported the titania–SWCNT work. Full details are available in the paper, “Photoinduced Charge Transfer and Acetone Sensitivity of Single-Walled Carbon Nanotube-Titanium Dioxide Hybrids,” by Mengning Ding, Dan C. Sorescu, and Alexander Star, J. Am. Chem. Soc. (DOI: 10.1021/ja402887v).
