
[Image above] Yang Yang (left), graduate student and first author; and Ju Li, right, professor of nuclear engineering and science and of materials science and engineering and corresponding author. Credit: Yang Yang
A big problem with most metals is that they eventually rust. And rust and corrosion can sometimes result in tragic consequences.
Back in 1967, stress corrosion, along with corrosion fatigue were responsible for claiming 46 lives in the collapse of the Silver Bridge.
Many people might remember the Aloha Airlines tragedy, when corrosion, once again, was the culprit.
More recently, corrosion was responsible for several injuries and two deaths at a Canadian mall in 2012.
Closer to home, last year a ride at the Ohio State Fair malfunctioned due to “excessive corrosion,” according to investigators in a CBS report.
Researchers everywhere have been working to solve corrosion challenges—from corrosion-resistant materials and coatings, to corrosion-resistant borides, to even predicting the potential for corrosion.
Scientists at Massachusetts Institute of Technology have discovered that a solid aluminum oxide protection layer can deform like a liquid when it’s applied to aluminum metal in a thin layer. The oxide could serve to protect metals from the environment—such as air and water—that contribute to degradation and corrosion. The oxide also could seal in gases and small molecules that need to be contained, such as hydrogen gas that powers fuel cell cars, or tritium that is produced inside the core of a nuclear power plant, according to an MIT news release.
Aluminum oxide, chromium oxide, and silicon dioxide all act as oxidation barriers. The research team wanted to further study the elements to see what makes them superior barriers. “We would like to understand the mystery why certain oxides (aluminum oxide and silicon oxide in particular) are good passivation layers, while others are not,” MIT professor of nuclear engineering and science and of materials science and engineering Ju Li explains in an email.
Led by graduate student Yang Yang, the researchers developed a unique method to observe what happens when surface oxides are exposed to oxygen and stress at atomic resolution. Using a special kind of transmission electron microscope—an environmental TEM (E-TEM) at Brookhaven National Laboratory, the team could evaluate the process in the presence of gases or liquids, instead of inside a vacuum, which is how samples are typically studied in a TEM.
Metal failure from stress corrosion cracking can happen even when the metal is surrounded by a protective layer. Cracks can still form, allowing air and other metal-corroding species access to the metal surface.
“We hope the oxide protection layer is liquid-like and can self-heal its cracks rapidly, Yang writes in an email. “It turns out the surface oxide of aluminum metal, one of the most common materials in our daily life, owns these special properties.”
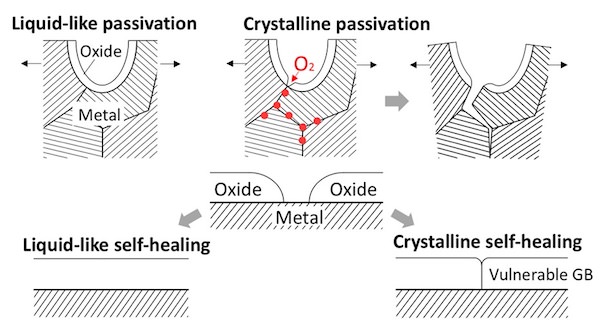
A comparison of liquid-like passivation vs. crystalline passivation. Reprinted with permission from (DOI: 10.1021/acs.nanolett.8b0006). Copyright (2018) American Chemical Society
Because no researcher had ever studied environmental deformation of metal oxides at atomic resolution, Yang’s team accomplished what no one else had—a thin sample of aluminum oxide deforming in oxygen gas with a thickness of 2–3 nanometers. “It is well-known that bulk metal oxide is very brittle,” Yang adds. “It is surprising that an ultrathin aluminum oxide layer can be so ductile, deforming like a liquid.”
Yang’s team also demonstrated that the aluminum oxide could be stretched more than twice its length without opening any cracks.
Their self-healing coating could be a solution the frustrating corrosion issues that have plagued structural engineers for years. Li says that in addition to nuclear power plants, their process could be used in other applications, such as “hydrogen generation, utilization, transport and storage,” he proposes.
The paper, published in Nano Letters is “Liquid-Like, Self-Healing Aluminum Oxide during Deformation at Room Temperature” (DOI: 10.1021/acs.nanolett.8b00068).
Watch the videos below to observe the aluminum oxide’s self-repair process.
Deformation of aluminum at moderate speed, showing that aluminum oxide can be kept elongated without fracture (Liquid-like behavior). Credit: Li Group, YouTube
A crack in aluminum oxide is self-repaired, forming a conformal and seamless oxide protection layer. Credit: Li Group, MIT, YouTube
Did you find this article interesting? Subscribe to the Ceramic Tech Today newsletter to continue to read more articles about the latest news in the ceramic and glass industry! Visit this link to get started.
