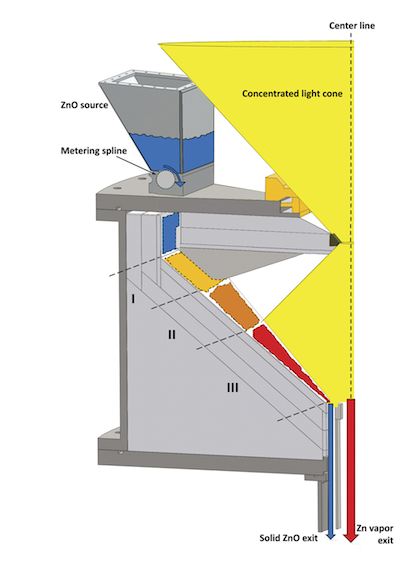
 Schematic diagram of one tile of solar reactor. Fifteen tiles arranged in a funnel shape comprise a solar reactor capable of reaching temperatures approaching 2,000 K, enough to dissociate zinc oxide. Zinc vapor is collected and condensed into micro- and nanoscale particles. Through hydrolysis, the zinc particles split water molecules and generate hydrogen. Credit: Prasad; University of Delaware.
Schematic diagram of one tile of solar reactor. Fifteen tiles arranged in a funnel shape comprise a solar reactor capable of reaching temperatures approaching 2,000 K, enough to dissociate zinc oxide. Zinc vapor is collected and condensed into micro- and nanoscale particles. Through hydrolysis, the zinc particles split water molecules and generate hydrogen. Credit: Prasad; University of Delaware.
Industrial scale solar installations need a lot of space and as much sunshine as possible.
We call such places deserts.
Unfortunately, though, most people find deserts unpleasant places to live.
One huge advantage of carbon-based energy sources is their transportability. Railroads and 18-wheelers constantly criss-cross the nation moving oil, natural gas, and coal from where they are plentiful (or processed) to where they are needed. One huge problem facing the hydrogen-based economy is that shipping the extremely light hydrogen is not practical.
Would it not be cool if solar energy could be trucked from the desert to somewhere it could be used—somewhere that people like to live and work? If you think about it, there is one resource that tends to be plentiful in areas where people do tend to settle and develop industry—water. Water, as I’ve mentioned before, is a great place to store hydrogen. Splitting the molecule apart is the tough part.
Getting these two energy resources together—sunshine and water—is the idea behind some new research coming out of the University of Delaware, where mechanical engineering professor Ajay Prasad and his group are making “solar fuel.”
This is not so much a materials engineering story as it is a materials exploitation story. Prasad and his graduate student, Erik Koepf, use basic thermodynamic principles to dissociate zinc oxide and precipitate zinc metal granules. Later, zinc metal is reacted with water, where it happily oxidizes and liberates hydrogen, which can be used as fuel.
Prasad said in a phone interview that he sees the technology as a way to “make zinc centrally, and then generate hydrogen locally.” He noted that a single tubular semi-truck can carry only about 100 kilograms of hydrogen, but can transport several tons of zinc particles.
It takes a lot of energy to dissociate zinc oxide, though, and Prasad’s and Koepf’s work focuses on designing a solar reactor that can concentrate enough sunlight and reach high enough temperatures to drive the dissociation reaction.
According to a press release, Koepf successfully tested a solar reactor design last April. Prasad told me, “We are at the proof-of-concept stage. In the first round of testing last April, we couldn’t get high enough temperatures. The reason is that the [reflector] mirror was too small, and we were losing available energy.” The reflector mirror directs the light from the solar concentrators into the reaction chambers.
During the April test, reactor temperatures of about 1,200 degrees Celsius were reached. Temperatures of about 1,400 to 1,700 degrees Celsius (1,700 to 2,000 K) are desired for the dissociation reaction. Since then, Koepf has been reworking the reactor design, especially the mirror component to focus 5,000 to 10,000 suns of concentrated energy into the reactor. When I talked to Prasad, Koepf was literally boarding a plane to test the tweaked solar reactor at the Solar Technology Laboratory at the Paul Scherrer Institute in Switzerland.
If you guessed that the reactor has ceramic components, you are correct. The reactor is a funnel-shaped design made of 15 fitted, trapezoid-shaped alumina plates. (A schematic diagram of one of the plates is shown above.). At the top of each trapezoid is a hopper of zinc oxide powder, which is sprinkled into the funnel with a metering spline. The powders descend through the reactor just by gravity.
According to a paper by Koepf, et al. published last fall in the International Journal of Hydrogen Energy, the first layer of powder sinters on top of the alumina plates, and subsequent layers of powders dissociate as they descend. The sintered zinc oxide layer does not react with the alumina and is easily scraped off. (Images from the paper, including the reactor, can be viewed without purchasing the paper.)
As shown in the diagram, there are three distinct temperature zones, each of which corresponds to different heat transfer mechanisms. Zone I is the preheat region with temperatures of 1,300-1,650 K. In Zone II, temperatures reach 1,650-1,800 K under diffuse radiation. The dissociation reaction begins here. Finally Zone III experiences direct radiation and temperatures in the 1,800-1,900 K range. Dissociation finishes here. At the bottom of the funnel, unreacted zinc oxide drops out. A flowing argon atmosphere helps create a tornado-like environment in the chamber, as well as sweeping the zinc vapor into an alumina collection tube. There, the vapor is quenched very quickly to condense it before reoxidation can occur. Overall, the powders spend about one-half second in the reactor.
The hydrolysis reaction to oxidize zinc happens at about 600 degrees Celsius. So, even though this is not an “energy cheap” process, it is passive and offers a mechanism for distributing energy resources. Prasad imagines an industrial-scale operation wherein a desert-based field of a thousand solar concentrator mirrors focus on a solar reactor mounted on a tower, and tons of dissociated zinc are trucked to sun-poor, water-rich areas where, by setting up favorable thermodynamics, hydrogen is generated.
His work is funded by the Department of Transportation, Federal Transit Administration.
The paper is, “A novel beam-down, gravity-fed, solar thermochemical receiver/reactor for direct solid particle decomposition: Design, modeling, and experimentation,” by Erik Koepf, Suresh G. Advani, Aldo Steinfeld, and Ajay K. Prasad; International Journal of Hydrogen Energy. DOI: 10.1016/j.ijhydene.2012.08.086.
