Establishing and expanding new and existing mining operations is a complex process. Virginia-based Kyanite Mining Corporate recently published a three-part series on the complexities of mining, which included a discussion on the need to make mining sustainable.
Read MoreGlass-like knee grafts, MOFs for water testing, and other materials stories that may be of interest for April 24, 2024.
Read MoreCertain carbon nitride compounds are predicted to rival or surpass diamond in terms of hardness, but attempts to experimentally synthesize these compounds in the past 30 years have failed. Now, in a recent groundbreaking study, researchers report successful synthesis of four covalent carbon nitrides that can all be recovered at ambient conditions.
Read MoreThe 2024 Pan American Ceramics Congress and Ferroelectrics Meeting of Americas took place April 7–11 in Panama City, Panama. The conference witnessed a surge in attendance compared to 2022 and welcomed a record-number of students from South American countries.
Read MoreWhat gives colored glass its brilliant hues? Since the early days of alchemy, our understanding of and control over the design of colored glasses has improved enormously, opening the door to a host of practical applications.
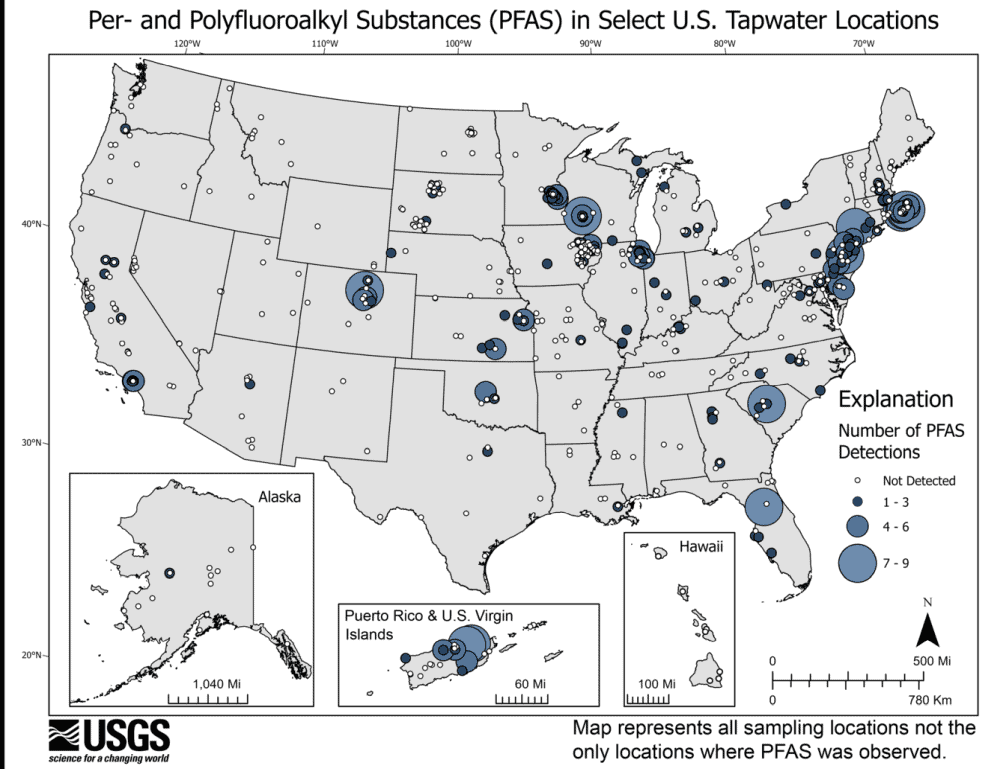
Read MoreOn April 10, 2024, the U.S. Environmental Protection Agency issued the first legally enforceable national drinking water standards for several common types of PFAS chemicals. Learn what the new standards mean for U.S. drinking water and how ceramics may play a role in the cleanup efforts.
Read MoreMagnetic nanographene butterfly, resistance-free electron channels, and other materials stories that may be of interest for April 17, 2024.
Read MoreThough the tradition of creating grand stained-glass windows is less common than it was before, the artform remains an important part of our culture today. In April 2024, CTT is running a special three-part series on stained glass. Part 2 provides an overview of the early history of colored glass and details the rise and fall of stained-glass windows between the 11th and 16th centuries.
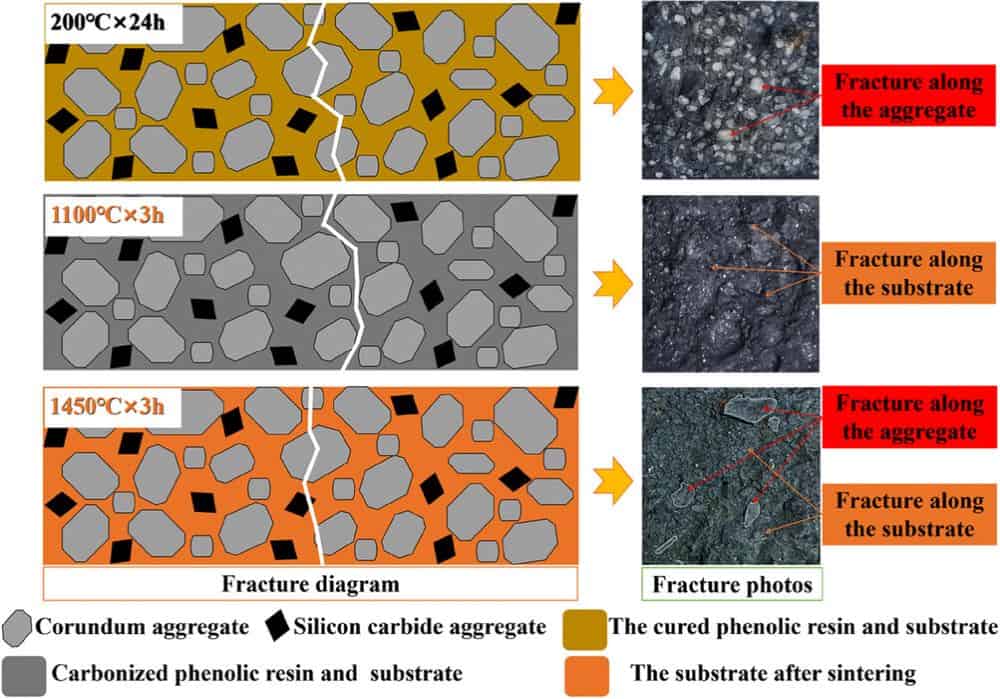
Read MoreThe choice of starting material for refractory ceramics can greatly affect the product’s final properties. Researchers from Wuhan University of Science and Technology in China investigated the effects of different corundum aggregates on Al2O3-SiC-C bricks.
Read MoreTo celebrate this year’s National Robotics Week, we have curated some of our past CTTs featuring fun innovations in robot technology.
Read More