ORNL researchers have developed a “double-duty” battery that not only can be made as small as a coin, but also can last for a decade at 26-percent higher capacity. Credit: ORNL.
What are you doing for the next 10 years?
Not quite sure? I get it. Many times I don’t what I’ll be doing in the next 10 minutes, let alone the next decade.
But no matter where the years might take us, we can keep calm and carry on without the need to recharge the devices that power us through our day (think smartphones and other energy-sucking smart gadgets and wearables), courtesy of a new battery developed by researchers at Oak Ridge National Laboratory.
The new battery prototype may help us bid bye-bye to charging cables and USBs, thanks to a “new and unconventional battery chemistry” that can sustain a single charge for up to 10 years.
According to an ORNL press release, their findings, published in the Journal of the American Chemical Society, “challenged a long-held assumption that a battery’s three main components—the positive cathode, negative anode, and ion-conducting electrolyte—can play only one role in the device.”
The electrolyte in the new design pulls “double-duty”—serving both as an ion conductor and cathode supplement—and is further enabled by ORNL’s solid electrolyte, which boosts the battery’s capacity and life.
“This bi-functional electrolyte revolutionizes the concept of conventional batteries and opens a new avenue for the design of batteries with unprecedented energy density,” says ORNL’s Chengdu Liang in the release.
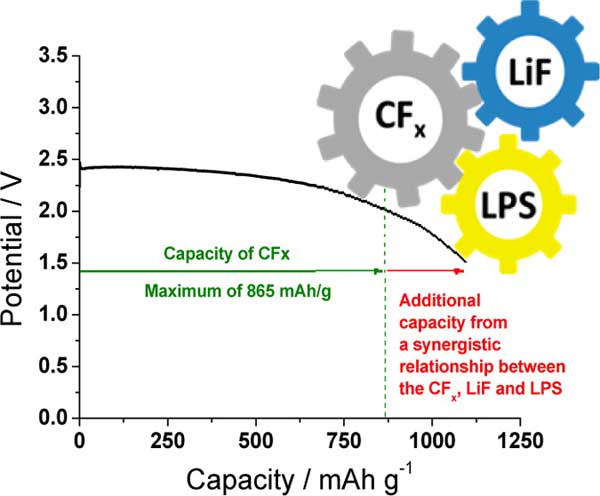
The ORNL team was able to demonstrate the concept in what’s considered to be “one of the best single-use batteries because of its high energy density, stability and long shelf life.” The solid lithium thiophosphate electrolyte incorporated into a lithium carbon fluoride battery resulted in a battery with an increased capacity of 26 percent.
“As the battery discharges, it generates a lithium fluoride salt that further catalyzes the electrochemical activity of the electrolyte,” Liang said. “This relationship converts the electrolyte—conventionally an inactive component in capacity—to an active one.”
The release notes that, depending on how the battery is engineered or used, the capacity improvement may equal years or decades of additional life and, given its size (reported to be as small or smaller than a coin), would be particularly useful in devices where easy or desirable battery replacement or recharging is neither easy nor desirable (artificial cardiac pacemakers, radiofrequency identification devices, and remote keyless systems and sensors).
According to ComputerWorld, don’t toss your single-use batteries or rechargeables just yet. The dual-functioning technology may make it to market within a few years, but whether that happens sooner rather than later is likely dependent on the cost to produce each battery.
The paper is “Pushing the theoretical limit of Li-CFx batteries: A tale of bifunctional electrolyte” (DOI: 10.1021/ja5026358).
Feature Image Credit: Paul Swansen on Flickr (Creative Commons License).