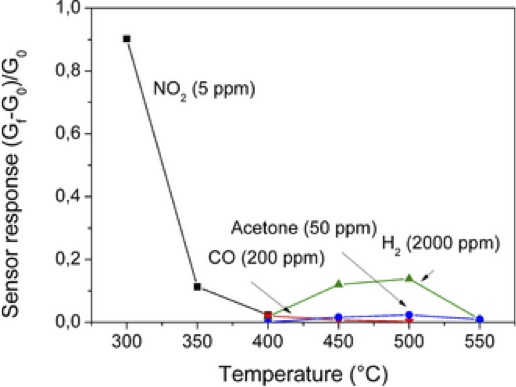
Sensitivity of glassy SiOC to several gasses: (▼) CO (200 ppm), (▲) H2 (2000 ppm), (●) Acetone vapor (50 ppm), and (■) NO2 (5 ppm).(Credit: Karakuscy; Wiley.)
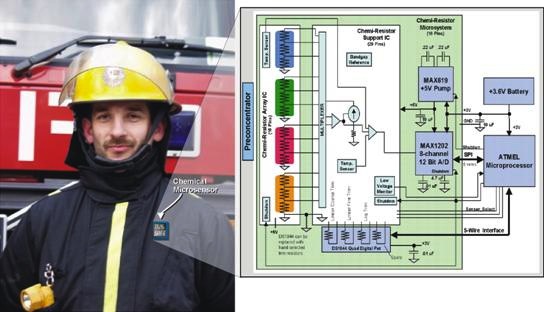
The August issue of the Journal of the American Ceramic Society includes an interesting little report in the Rapid Communications section titled, “Gas Sensing Behavior of Mesoporous SiOC Glasses,” by a two-university team in Italy. Because these mesoporous glasses are semiconducting, they could be useful as chemiresistors for detecting tiny amounts of gaseous species. Applications for these types of devices include sensing in remote locations (e.g., downwell bores) or hazardous environments. The low power requirements of the devices makes them attractive to organizations like Sandia National Laboratories, which are looking into incorporating chemiresistors into the badges of first responders (see image above from the Sandia website), for example. There is also some interesting work on using this type of technology for medical diagnostics, too. (Sandia has a short video illustrating the principle of chemisresistance.)
In this work, the glasses start as “ambigels”—ambient dried aerogels—made by a sol-gel process from preceramic polymers and pyrolyzed to form glassy SiOC. The gels are highly porous and therefore have a very high surface area, and so do the glasses—the paper reports BET specific surface area of 150m2/g with a total volume of 0.19 cm3/g for SiOC after pyrolyzing at 1,400°C. The mesoporous structure ranges from 2–20 nm in a distribution that peaks at 10 nm. The paper notes that the open porosity after pyrolyzing at 1,400°C for three hours is remarkable given that pure silica gel starts to sinter above 1,000°C.
The process lends itself to a wide composition range from stoichiometric SiOC to carbon-rich SiOC compositions defined as SiCxO4-x where x ranges from 0–4. The resulting structure is a complex mix of glassy structure of varying composition, nanocrystalline SiC, and interconnected graphene layers. The amount of free carbon is key, according to the paper, because the conductivity of the glass “increases with increasing free carbon content due to the formation of a percolation network of precipitated turbostratic carbon.”
The group tested the sensors for sensitivity over a range of temperatures to four gasses: NO2 at 5–10 ppm; CO at 200–500 ppm; H2 at 2,000–5,000 ppm, and acetone at 50–100 ppm. They also looked at sensor performance in humid atmospheres.
As the graph above shows, the sensors exhibited two response regimes. Below 400°C, they respond well to NO2. Above 400°C, sensitivity to NO2 disappears, and they become responsive to H2, although the hydrogen concentration is much higher—2,000 ppm, versus 5 ppm for NO2.. The material is effectively nonresponsive to CO and acetone, at least at the studied temperatures and concentrations.
The authors say the material’s response to both reducing and oxidizing environments shows that it cannot be classified as either a n-type or p-type semiconductor. However, this dual behavior has been observed in other polymer-derived ceramic materials, which have both electron and hole charge carriers. Continuing studies are underway to uncover the conduction mechanism.
The paper is “Gas Sensing Behavior of Mesoporous SiOC Glasses,” by Aylin Karakuscu, Andrea Ponzoni, Parakkulam R. Aravind, Giorgio Sberveglieri, and Gian D. Soraru; Journal of the American Ceramic Society. DOI: 10.1111/jace.12491.