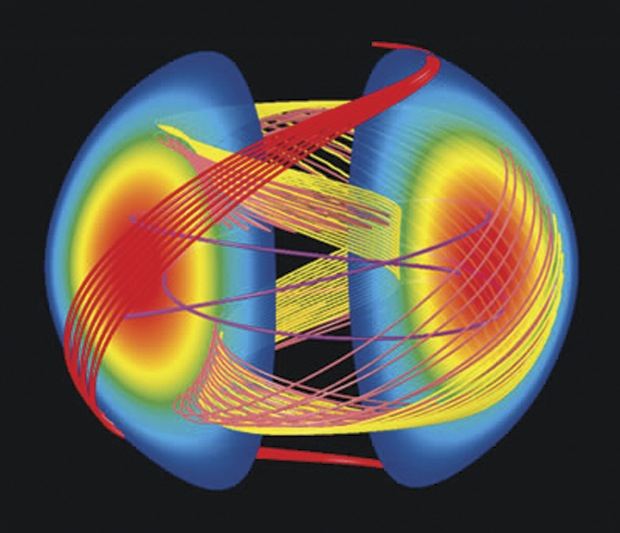
Berkeley Lab researchers increase NMR/MRI sensitivity through hyperpolarization of nuclei in diamond
Today’s nuclear magnetic resonance and magnetic resonance imaging technologies, like quantum information processing and nuclear spintronic technologies, are based on an intrinsic quantum property of electrons and atomic nuclei called “spin.” Electrons and nuclei can act like tiny bar magnets with a spin that is assigned a directional state of either “up” or “down.” NMR/MRI signals depend upon a majority of nuclear spins being polarized to point in one direction. The greater the polarization, the stronger the signal. Researchers with the DOE’s Lawrence Berkeley National Laboratory have reported on a technique for hyperpolarizing carbon-13 nuclear spins in diamond that enhances the sensitivity of NMR/MRI by many orders of magnitude above what is ordinarily possible with conventional NMR magnets at room temperature. The work builds upon earlier research by several groups worldwide, and suggests a route by which the sensitivity of generic NMR and MRI experiments can be enhanced in applications related to molecular and biomolecular detection, diamond-based quantum information processing, and nuclear spintronics.
Fraunhofer ISE, EV GROUP team for direct semiconductor wafer bonds for next-generation solar cells
The Fraunhofer Institute for Solar Energy Systems and EV Group (EVG) will jointly develop equipment and process technology to enable electrically conductive and optically transparent direct wafer bonds at room temperature. The work aims to enable highly mismatched material combinations like gallium arsenide on silicon, GaAs on indium phosphide, InP on germanium, and GaAs on gallium antimonide. Direct wafer bonding provides the ability to combine a variety of materials with optimal properties for integration into multi-junction solar cells, which can lead to new device architectures with unparalleled performance, according to Fraunhofer ISE. Direct wafer-bonding is expected to play an important role in the development of next-generation III-V solar cell devices with applications in space as well as in terrestrial concentrator photovoltaics.
A team led by researchers at Oxford University’s Department of Engineering Science and The Oxford Martin School has been pushing the boundaries of what electric vehicles can do with their prototype EV PEGGIE. After a successful debut at last year’s Shell Eco-marathon Europe competition, the team entered this year’s event in Rotterdam on May 19 and won the Technical Innovation Award ahead of nearly 200 other teams from across Europe. This year the car sported a photovoltaic array of 130 individual cells which continually reconfigure themselves for maximum efficiency, improving overall efficiency by more than five percent. The design also enables regenerative braking and free-wheeling, and features a “smart” clutch that allows the entire drivetrain to be built from smaller and lighter components. Finally, an Android phone app for the driver delivers a colorful plot of torque along one axis and speed along another, allowing maximum efficiency regardless of conditions.
Elaborate nanostructures blossom from a chemical reaction
Harvard University researchers have created minuscule sculptures, curved and delicate, that don’t resemble the cubic or jagged forms normally associated with crystals, though that’s what they are. Rather, fields of carnations and marigolds seem to bloom from the surface of a submerged glass slide, assembling themselves a molecule at a time. By simply manipulating chemical gradients in a beaker of fluid, the scientists at Harvard’s School of Engineering and Applied Sciences can control crystal growth to create precisely tailored structures. Crystal precipitation depends on a reaction of compounds that are diffusing through a liquid solution. The crystals grow toward or away from chemical gradients as the pH of the reaction shifts back and forth. Conditions dictate the structure’s geometry. To create the flower structures, the researchers dissolve barium chloride and sodium silicate (waterglass) into a beaker of water. Carbon dioxide from air naturally dissolves in the water, setting off a reaction that precipitates barium carbonate crystals. It also lowers the pH of the solution immediately surrounding the crystals, which then triggers a reaction with the dissolved waterglass to produce a layer of silica on the growing structures, uses up the acid from the solution, and allows the formation of barium carbonate crystals to continue.
Confirmed: metallic glasses based on icosahedral clusters
A defining property of any material considered a “glass” is its amorphous structure, right? Well… Using a new electron scattering technique and one of the world’s highest-resolution electron microscopes, scientists at Monash University (Victoria, NSW, Australia) found the structure of a zirconium-based metallic glass was composed mainly of 13-atom icosohedral clusters. With 20 faces, 12 vertices, and 12 axes of five-fold symmetry, the structures cannot be packed into an ordered 3D crystalline structure. Researchers also expect the new technique to be useful for understanding the structure of other disordered materials.
Insulator + insulator = superconductive, magnetic material
Scientists using finely tuned X-rays at the Stanford Synchrotron Radiation Lightsource (SSRL; Stanford, Calif.) have detected magnetism at the interface of two insulating, nonmagnetic materials. When the researchers sandwiched together two perovskite minerals, lanthanum aluminum oxide and strontium titanium oxide, they discovered the resulting “heterostructure” was both superconductive and had magnetic qualities—an unusual combination of properties that neither material has alone. The workers proved the magnetism comes from the titanium atom, although further study is needed to determine the mechanism that caused the material to become magnetic.
