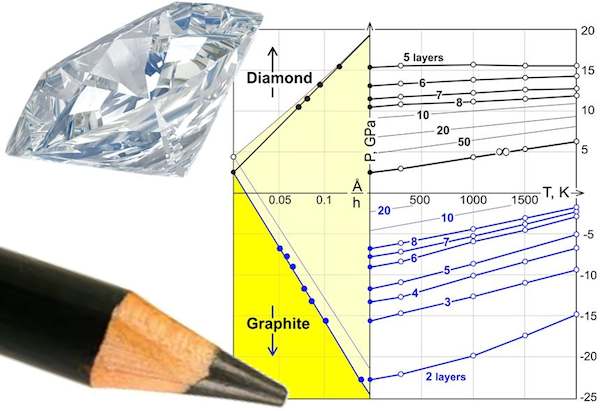
Scientists created a phase diagram for the chemical transition of thin graphene layers to diamond. (Credit: Sorokin; Technological Institute for Superhard and Novel Carbon Materials)
Besides being a girl’s best friend, diamond has some excellent and unique material properties—mechanical strength, optical transparency, high thermal conductivity, electrical insulation, and potential semiconductor properties, to name a few.
Because of these properties, creating nanometer thin diamond films is a hot topic of research—just think of all the potential applications!
While it is possible to convert thin sheets of graphene into diamond, or “diamene,” scientists previously thought this transition could only occur by applying huge amounts of pressure. But now, a group of scientists from Rice University and Russia has theorized that it is possible to chemically convert the material, eliminating the need for immense pressure (which the authors calculated to be 12 GPa).
The paper, published in Nano Letters, describes how the group modeled the interactions between individual atoms in a layer of graphene to develop a phase diagram for the transition of thin stacked layers of graphene to diamene. Previous work has created phase diagrams for the transition in bulk materials, but new interest in single layers of diamene—which are so thin they are considered two-dimensional—necessitated a recalculation.
According to a Rice University press release, paper coauthor Boris Yakobson said: “A phase diagram shows you which phase dominates the ground state for each pressure and temperature. In the case of diamane, the diagram is unusual because the result also depends on thickness, the number of layers of graphene. So we have a new parameter.” Yakobson is professor of mechanical engineering and materials science and professor of chemistry at Rice.
The new phase diagram details the conditions under which graphene transforms to diamene, providing a recipe for future success. The diagram also suggests it is possible to chemically transform graphene into diamene, under the right conditions, with little or no pressure required. In this situation, hydrogenation of the graphene surface facilitates the transition from rags to riches.
“When the hydrogen attacks, it takes one electron from a carbon atom in graphene,” Yakobson said in the press release. “As a result, a bond is broken and another electron is left hanging on the other side of the graphene layer. It’s now free to connect to a carbon atom on the adjacent sheet with little or no pressure.
“If you have several layers, you get a domino effect, where hydrogen starts a reaction on top and it propagates through the bonded carbon system. Once it zips all the way through, the phase transition is complete and the crystal structure is that of diamond,” he said.
While the group has yet to produce the diamond thin films from graphene, the study marks an important milestone towards facilitating synthesis. Diamond thin films have a variety of potential applications, including films for electronics, optical materials, and cutting tools.
The paper is titled “Phase diagram of quasi-two-dimensional carbon, from graphene to diamond.” DOI: 10.1021/nl403938g.
