 [Image above] Credit: Harvard John A. Paulson School of Engineering and Applied Sciences; YouTube
[Image above] Credit: Harvard John A. Paulson School of Engineering and Applied Sciences; YouTube
I’ve sung the praises of solid oxide fuel cells before.
And after a team in Germany set a new world record last year for the longest continually running cell, it seemed like those praises were fitting.
But, despite the success of that German record breaker, one problem that still plagues and limits fuel cells is that they degrade over time.
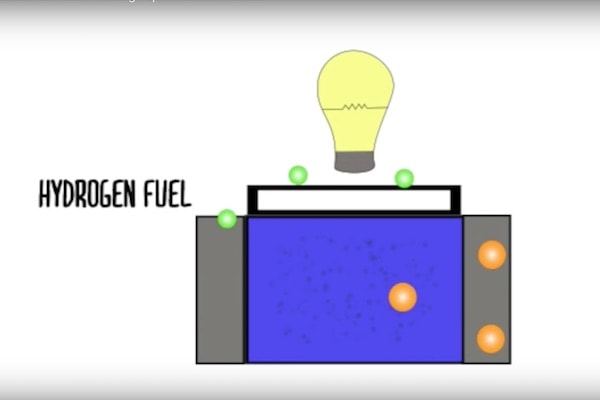
This problem is the result of the cell’s fuel—usually hydrogen—reacting with the electrolyte, disrupting its selective permissivity to protons and electrons. That selective permissivity is key to generating an electrical current within the cell, because it keeps charges separated. And charge separation translates into energy generation.
Researchers at the University of Connecticut introduced their solution to this problem earlier this year—a chromium capture technique that uses complex oxides to sequester chromium and prevent poisoning within a fuel cell.
Now, researchers at Harvard University have devised a different way to generate better fuel cells, this time using quantum materials.
“We have combined the fields of quantum matter and electrochemistry in a way that led to discovery of a new, high-performance material that can phase transition from a metal to ion conductor,” researcher Shriram Ramanathan says in a Harvard press release. Ramanathan is currently a professor of engineering at Purdue University, but performed the work as a visiting scholar in materials science and mechanical engineering at Harvard’s John A. Paulson School of Engineering and Applied Sciences.
Watch this short video to learn more.

Credit: Harvard John A. Paulson School of Engineering and Applied Sciences; YouTube
Ramanathan and graduate student You Zhou created a fuel cell electrolyte from a pervoskite-structured nickelate—which on its own isn’t picky when it comes to letting electrons or ions pass through.
But coating the nickelate with a catalyst and doping it with electrons that join the nickel ions’ electron shell makes the material an exclusively ion conductor, according to the release.
Suppressing the movement of electrons allows ions to move quickly through the electrolyte, while also suppressing electron leakage within the cell. That means better energy generation and less degradation over time.
According to the paper’s abstract, “The ionic conductivity of the nickelate perovskite is comparable to the best-performing solid electrolytes in the same temperature range, with a very low activation energy.”
Instead of degrading the electrolyte, exposure to fuel makes this new quantum-designed electrolyte more robust, according to the release.
“The elegance of this process is that it happens naturally when exposed to the electrons in fuel,” Ramananthan says. “This technique can be applied to other electrochemical devices to make it more robust. It’s like chess—before we could only play with pawns and bishops, tools that could move in limited directions. Now, we’re playing with the queen.”
The research, published in Nature, is “Strongly correlated perovskite fuel cells” (DOI: 10.1038/nature17653).
Author
April Gocha
CTT Categories
- Energy
- Material Innovations
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026
Solid-state batteries turn heads at CES 2026
January 29, 2026