Zeolite pellets are being used by Fraunhofer researchers in a new high-efficiency thermal energy storage system.
This is totally an empirical observation, but it seems like recently there have been a growing number of references to zeolites in science and technology literature. Maybe it’s not true, but given materials science’s keen interest on understanding and exploiting surface phenomena and microstructures (zeolites have enormous surface areas, are porous and easily engage in ion exchange), it doesn’t surprise me that processes and applications that exploit zeolite characteristics keep popping up.
Here are just a few examples I ran into this week:
New zeolite-based sorptive thermal storage system: Researchers from the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB in Stuttgart are developing a thermal storage system that uses zeolite pellets (see above), which the group says can store three to four times the amount of heat that water, alone, can. Thus, the zeolite systems can either be smaller … or used to store considerably more heat. Another benefit, according to Fraunhofer, is the the zeolite system is extremely efficient at storing heat for long periods of time (the group says the system is nearly loss-free) and can tolerate temperatures over 100°C. The system exploits the heat-exchange capability of zeolite when the material comes in contact with water. In this case, excess heat from, say, a power generation system, is applied to the pellets.
This process drives water vapor out of the pellets and energy is stored, although the pellets are not necessarily hot. They claim that if the dried zeolite material is prevented from coming into contact with water, it can store the heat for an unlimited amount of time. The process can be reversed and heat can be released by exposing the pellets to water or water vapor.
The group has developed several sizes of models of their system, the largest being a storage volume of 750 liters that is being tested and demonstrated on a mobile platform. They believe the system ultimately can be adapted to both industrial installations and small combined heat and power plants that are found frequently in large residential buildings in Europe.
Portable cooling vests and pads for cardiac patients: At the other end of the zeolite/water thermal properties spectrum is an application developed by another German group, this time at the Hohenstein Institute. The concept is based on the idea that one effective treatment for someone who goes into cardiac arrest is to limit neurological damage by rapidly lowering body temperature until proper heart function can be restored. The Hohenstein group designed a vest containing specially sealed “cooling pads” composed of a textile hollow fabric containing water. The vest is connected via a closed valve to a container of dry zeolite material that has been kept under a water-tight vacuum.
To be put into use, the vest is placed on the patient and the valve connecting the vest and the zeolite tank is opened. As the zeolite adsorbs the water vapor from the cooling pads, the overall temperature of the water in the system drops and the cooling effect of the vest quickly takes place. No external power is required for the cooling system to work. The researchers hope the self-sufficent system can be made inexpensively and be used to complement mobile defibrillators for use by paramedics and in first-aid situations.
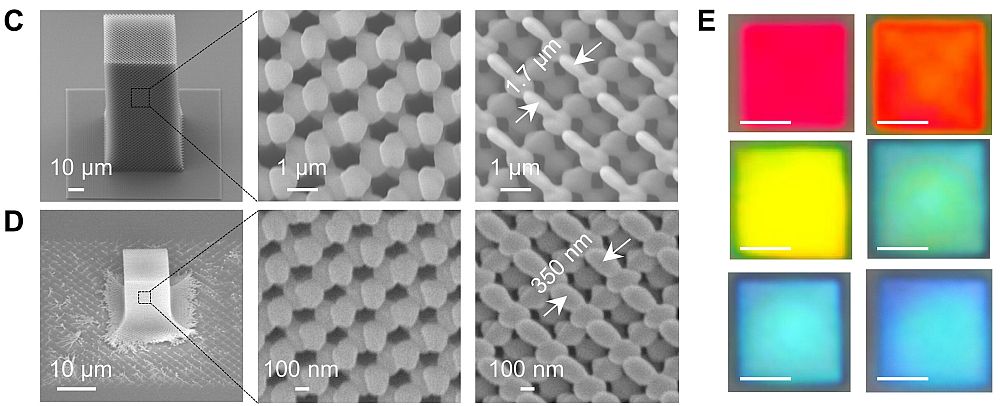
Nanoscale “house of cards” of catalytic zeolites. My third example is of a new technique for creating unique nanostructures from catalytic zeolites that are projected to improve the performance of catalysts in many fields, including fuels, chemical and pharmaceuticals. The technique is being developed by an international group led by researchers at the University of Minnesota and a report on the effort is in a new issue of Science. As mentioned above, zeolites naturally have an enormous surface area, and previously, the researchers had learned how to optimize the surface area of a catalytic class of zeolites by creating ultrathin nanosheets whose pore structure and size could be manipulated to allow them to serve as “specialized molecular sieves.”
In their newest work, the researchers show how they were able to form zeolite nanosheets with 0.5 nanometer pores that grow on connecting planes at 90-degree angles. As the illustration shows, the 3D house-of-cards-like structure of the nanosheets creates a permanent network of channels 2–7 nanometers wide. The effect of the increased surface area combined with the controlled size of the pores and channels led to faster access to catalysts (and, therefore, improved catalytic reaction rates), especially for larger molecules that tended to get somewhat “stuck” and slowed down in other catalytic materials. It is projected that the faster catalysts could significantly reduce chemical processing costs.
Finally, it should be noted that zeolites also are used in key processes in many other important industries, including nuclear, water treatment, construction and agriculture, and given this range of applications and the novel engineering breakthroughs that now are being made, it is a type of material that may just be coming into its prime years.
CTT Categories
- Energy
- Material Innovations
- Nanomaterials
- Thermal management