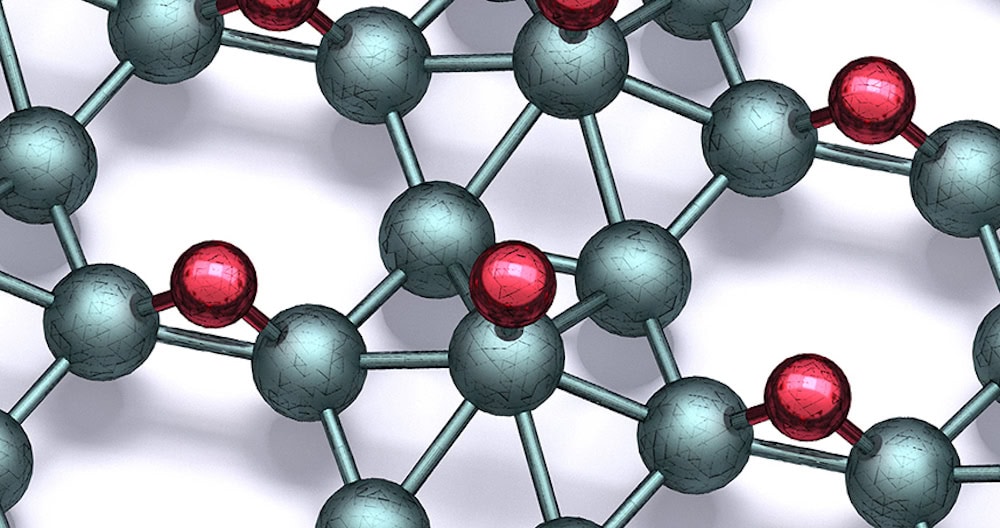
[Image above] Hydrogenating the 2D material borophene offers a way to stabilize it against ambient oxidation. In this image, grayish teal balls represent boron and red balls represent hydrogen. Credit: Mark Hersam, Northwestern University
As much as I enjoy writing about graphene, there’s a similar 2D material that really piques my interest—borophene.
Borophene, a 2D sheet of boron atoms, was first theorized in the 1990s and experimentally confirmed in 2015. Much like graphene, borophene is flexible, strong, and lightweight. It also forms in several different crystal structures (polymorphs), so structural manipulation of borophene is easier than it is for graphene. For these reasons, some feel borophene may serve certain applications such as wearable device applications better than graphene.
However, borophene rapidly oxidizes in air, which means integrating borophene into practical devices is difficult. To date, experimental characterization of borophene has required ultrahigh-vacuum conditions to avoid unwanted reactions with air.
Fortunately, passivation may address borophene’s volatility. Passivation refers to processes used to reduce the chemical reactivity of a material. Hydrogenation, or reaction with hydrogen, is one type of passivation process that first-principles calculations suggest could be used to stabilize borophene and suppress ambient oxidation.
In a recent study, Northwestern University researchers, along with colleagues from the University of Florida and Argonne National Laboratory, investigated the synthesis of hydrogenated borophene, or “borophane.” In the introduction, they explain that while theoretical explorations of borophane have taken place, “atomically well-defined synthesis and characterization of borophane polymorphs have not yet been achieved.”
To create borophane, the researchers exposed borophene to atomic hydrogen in ultrahigh-vacuum conditions. Then, they extensively studied the bonding structure and properties by combining scanning tunneling microscopy and spectroscopy, inelastic electron tunneling spectroscopy, and density functional theory.
Similar to the high degree of polymorphism in borophene, the researchers observed eight different borophane polymorphs. All of the polymorphs demonstrated markedly improved chemical and morphological stability in ambient conditions. In particular, degradation in borophane was only detected after 1 week of ambient exposure.
“This oxidation peak is clearly smaller than that of the borophene sample after 1 hour of ambient exposure, indicating that the oxidation rate is reduced by more than two orders of magnitude,” they write. These findings show “that diverse hydrogen-bonding motifs on borophene impart ambient stability.”
In addition to providing ambient stability on the order of days, which is a sufficient time window for most ambient characterization and processing methods, the researchers also found that the hydrogenation of borophene was reversible using thermal annealing.
“Specifically, borophane samples could be recovered to pristine borophene without apparent degradation after annealing the sample to ~300°C,” they write. This finding is significant because it means pristine borophene can be regained once ambient processing is complete or robust encapsulation layers are applied.
In a Northwestern University press release, senior author and Walter P. Murphy Professor of Materials Science and Engineering Mark C. Hersam says their findings will allow researchers to more rapidly explore borophane’s properties and its potential applications.
“Materials synthesis is a bit like baking,” he says. “Once you know the recipe, it’s not hard to replicate. However, if your recipe is just a little off, then the final product can flop terribly. By sharing the optimal recipe for borophane with the world, we anticipate that its use will rapidly proliferate.”
The paper, published in Science, is “Synthesis of borophane polymorphs through hydrogenation of borophene” (DOI: 10.1126/science.abg1874).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Nanomaterials
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026