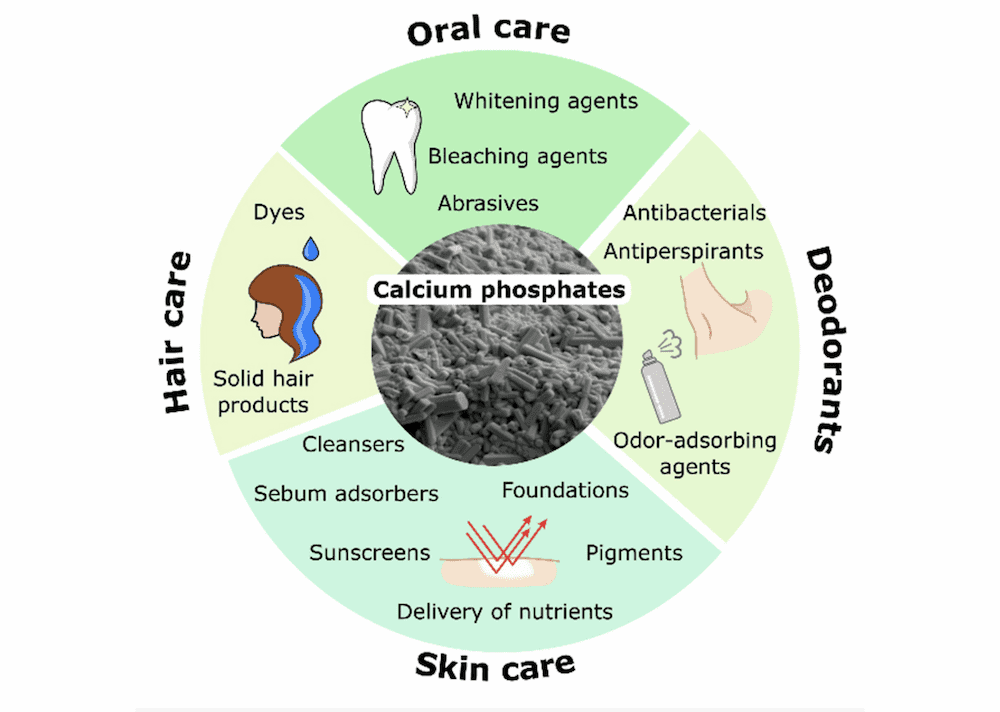
[Image above] Current and potential applications of calcium phosphates in the cosmetics industry. Credit: Carella et al., Materials
I’ve never been much of a makeup gal, but one recent piece of cosmetics news that caught my eye is the approval of milestone legislation in Mexico banning manufacture, import, and marketing of cosmetics tested on animals. Mexico is the first country in North America to pass such a ban, and it joins 40 other countries globally in doing so.
While most would agree that moving away from animal testing for cosmetics is morally agreeable considering the distress and pain such tests can inflict on animals, some worry that banning these tests will increase the chances of unsafe or hazardous products making their way to market.
However, bans on animal testing do not exist in a vacuum—countries that have passed animal testing bans, such as in the European Union, have numerous other pieces of legislation to ensure safety of products even in the absence of animal testing. Plus, bans can include exceptions—the Mexico legislation allows for animal testing when there is no alternative scientifically validated testing method.
The Three R’s principle has been instrumental in helping countries implement animal testing bans. The R’s stand for replacement, reduction, and refinement, and they provide a framework for more humane animal research.
With impetus from the three R’s, scientists have developed alternative tests that safely replace many animal tests. In addition, some companies negate the need for testing by using established ingredients with a history of safe use that do not require more testing.
Calcium phosphate is one ingredient that the nonprofit Cosmetic Ingredient Review program has concluded is safe for use when formulated to be nonirritating.
Calcium phosphate is a family of minerals containing calcium ions together with inorganic phosphate anions, and sometimes hydroxide ions as well. It is the main mineral in human bone and teeth, and it is considered highly biocompatible.
The main application of calcium phosphate is in medicine, where it is used to regenerate or replace bone tissue. Recently, the cosmetics industry has taken an interest in this mineral family as well, and a new open-access paper reviews what the industry has used calcium phosphate for so far.
Researchers from the National Research Council Institute of Science and Technology for Ceramics in Faenza, Italy, wrote the 37-page paper. Below are highlights from the review.
Applications of calcium phosphates in cosmetics
The authors identified four phases of calcium phosphate used in cosmetic applications.
- Amorphous calcium phosphate is a noncrystalline phase that serves as a precursor for bone and tooth formation. It is more soluble than crystalline calcium phosphates and so is used in the dental field to develop ion-releasing toothpastes that trigger enamel and dentin remineralization.
- Hydroxyapatite [Ca10(PO4)6(OH)2] is the most thermodynamically stable calcium phosphate phase. It is found in vertebrate bones, mammalian teeth, and fish scales, and there is a wide array of methods for its preparation.
- Octacalcium phosphate [Ca8H2(PO4)·6.5H2O] can convert into hydroxyapatite through a dissolution–reprecipitation mechanism or a topotaxial conversion mechanism. For this reason, it has found successful application in bone-related biomaterials.
- Tricalcium phosphate [Ca3(PO4)2] exists in two allotropic forms that have the same chemical composition but different structure, density, and solubility: α-TCP and β-TCP. The main medical application of tricalcium phosphate, in particular α-TCP, is as self-setting calcium phosphate cements.
Based on a scientific literature review and patent search, the authors identified four main applications of calcium phosphates in cosmetics: oral care, skin care, hair care, and deodorants.
Oral care
Several products for tooth whitening exist on the market and can be divided into two categories: bleaching agents and abrasives.
Current whitening products face some drawbacks. The strong oxidation of bleaching agents can degrade the organic matrix of enamel and dentin, while their acidic components damage the mineral component. Regarding abrasives, their action is limited by the accessibility of the toothbrush to stained areas of the teeth, and prolonged use can significantly wear enamel and dentin.
Calcium phosphate may overcome some of these limitations. Studies demonstrate that the remineralization action of calcium phosphate makes the enamel surface smoother and less prone to staining.
However, based on the published papers, it is not possible to assess whether calcium phosphate is more efficient than any other whitening agent because “these works have studied the whitening effect of [calcium phosphate] as a raw material, as a prototype gel or toothpaste formulation, or as a commercial product, making it very difficult to discriminate the influence of [hydroxyapatite] on the whitening effect from other ingredients,” the authors write.
On the other hand, some studies show that the combination of calcium phosphate with bleaching agents improves tooth whiteness and at the same time prevents enamel demineralization, hardness loss, tooth sensitization, and surface roughening.
Skin care
Most studies in this area focus on the use of calcium phosphates as sunscreens, cleansers, and makeup products.
Several studies show that materials commonly used as UV filters in sunscreens contaminate almost all water sources around the world, and removal of these substances using typical wastewater treatment techniques is very difficult.
Hydroxyapatite could serve as a safe alternative UV filter, according to several examples in the literature. The main challenge of using either synthetic or natural calcium phosphate in sunscreen is the material’s intrinsic UV absorption limit, but doping the calcium phosphate with metal cations, for example, can improve this limit.
Skin cleansing is a well-explored cosmetic application of calcium phosphate, with the authors identifying 17 published patents. Almost all patents harness the high adsorption capability of calcium phosphate to adsorb sebum, while the others use calcium phosphate to promote skin turnover or clean the skin through the mineral’s abrasive action.
Calcium phosphate serves several different purposes in makeup, according to an analysis of patents. Several patents used calcium phosphate as an enhancer for conventional makeup products, imparting an anti-sebum effect due to adsorption action. Calcium phosphate was also used for pigment purposes in some cases, such as to impart a white color, to host colored cerium phosphors, or to stabilize oil-soluble dyes.
Hair care
Compared to the other applications, use of calcium phosphate as a hair care ingredient is not common. The authors found only two relevant patents, where the application of calcium phosphate was as a dye carrier inside temporary hair dye products. In both cases, “hydroxyapatite was used thanks to its ability to encapsulate water-insoluble ingredients,” they write.
Deodorants
Deodorant ingredients can be divided into three categories based on the method used to control odor: antiperspirants, which suppress production of sweat by physically blocking the ducts; antimicrobials, which inhibit bacterial activity; and fragrances, which cover unpleasant smells.
Recently, researchers proposed a new category of deodorant ingredients: adsorbing materials, which suppress unpleasant smells by adsorbing volatile malodorous substances through noncovalent interactions.
Biomedical research shows that calcium phosphate can adsorb a wide variety of organic molecules, and the presence of both positive and negative charges on the calcium phosphate surface allows for adsorbing both cationic and anionic molecules.
“Despite these promising features, the use of calcium phosphate as an adsorbent for malodorous molecules is still in its infancy and the number of research articles on this application is scarce,” the authors write.
Suggestions for future research
In the conclusion, the authors discuss some of the directions they believe future cosmetics research on calcium phosphate will take.
For tooth whitening, they suggest the focus will be on development of more efficient remineralization materials than on bleaching ones.
For skin care applications, they propose there will be more consideration for the use of calcium phosphate as “SPF boosters” in sunscreen. Plus, they feel the abrasive and adsorbent properties of calcium phosphates will be further optimized.
“Furthermore, nanometric [calcium phosphate] can act as biocompatible stabilizers for Pickering emulsions and could substitute the currently used emulsifying agents,” they add.
For hair care, they write that calcium phosphate can act as vehicles to deliver dyes, nutrients, and other useful molecules to the follicle and to hair skin.
For deodorants, they suggest that future studies could tune the surface chemistry of calcium phosphate to enhance sorption properties. “In addition, the capability of [calcium phosphate nanoparticles] to act as antiperspirants through pore blocking should be studied in more detail,” they add.
They conclude by highlighting another opportunity they feel has been neglected until now—the use of alternative calcium phosphate crystal phases to hydroxyapatite.
“Indeed, almost all of the research works reported in this review have been focused on [hydroxyapatite], as it is the most used material in medicine and cosmetics. Different [calcium phosphate] phases have different properties, such as solubility, morphology, and surface chemistry, that could make them attractive for specific applications,” they write.
The open-access paper, published in Materials, is “The use of calcium phosphates in cosmetics, state of the art and future perspectives” (DOI: 10.3390/ma14216398).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Education
- Material Innovations
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026
Ohio Creativity Trail: Heisey Glass Museum
January 13, 2026


