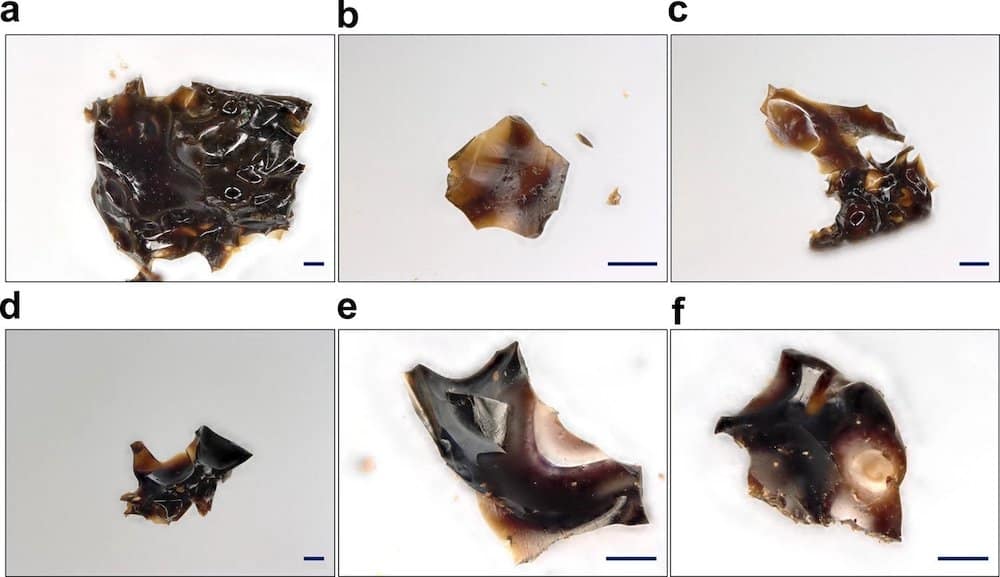
[Image above] Examples of ionic liquid-incorporated ZIF-8 showing evidence of melting and glass formation after treatment in low temperature (a–c) and high temperature (d–f) conditions. Scale bars are 100 µm. Credit: Nozari et al., Nature Communications (CC BY 4.0)
In the search for next-generation energy and environmental technologies, metal-organic frameworks (MOFs) are one material class that shows up regularly in the literature.
MOFs are highly porous crystalline compounds composed of highly ordered 3D networks of metal ions and organic ligands. MOFs are of great interest to researchers due to the material’s porosity, which allows diffusion of guest molecules into the bulk structure and thus makes MOFs ideal for storage, separation, and catalysis applications.
To date, MOFs have not achieved commercial viability due to difficulties synthesizing these materials in bulk form. The relatively weak metal–ligand coordination bonds in MOFs make the materials chemically unstable, thus giving them low endurance in different types of chemical environments.
The discovery a few years ago that forming MOFs in a glassy phase gives them enough stability for bulk production was a milestone, and there are now several studies on the properties of such glasses.
Researchers have devoted particular interest to MOF glasses made from zeolitic imidazolate frameworks (ZIFs). ZIF-based MOFs exhibit higher thermal and chemical stability than other MOF subsets.
Frustratingly, only a handful of ZIFs have led to melt-quenched glasses due to the limited meltability of many ZIFs, which is a result of the decomposition temperature being lower than the melting temperature. In other words, the organic ligands decompose before the metal–ligand coordination bonds can break and reform, preventing the material from reaching the liquid state needed for glass formation.
If the material could be modified so that the melting temperature is reached before decomposition, it would enable access to a much more diverse array of MOF glasses. Fortunately, strategies exist for decreasing the melting temperature of a material, and researchers of a recent open-access paper demonstrate the potential of one such method on ZIF-8, a commercially available ZIF with sodalite topology.
The researchers come from the University of Jena in Germany and the University of Cambridge in the United Kingdom. In their paper, they explain that the relatively high porosity of ZIF-8 makes the material favor thermal decomposition. However, ionic liquid offers a possible way to counteract this tendency.
Ionic liquids are salts that exist as liquid at temperatures below 100°C. Researchers have conducted extensive experimental and computational investigations on ionic liquid–MOF composites because interaction between these materials can create new functional sites favorable for adsorption, catalysis, and ion conduction.
In 2017, a paper by researchers in Turkey clarified another result of the ionic liquid–MOF interaction. Specifically, they showed that the ionic liquid weakened the bond between the organic ligands and metal ions, resulting in lower thermal stability—and thus making the composite easier to melt.
The possibility that ionic liquids may make certain MOFs easier to melt is what the researchers of the new study explored. They chose the hydrophobic ionic liquid [EMIM][TFSI] and loaded it into ZIF-8 using a wet impregnation technique.
After confirming through X-ray diffraction and scanning electron microscopy that incorporating the ionic liquid did not damage the crystal structure or morphology of ZIF-8, the researchers used thermogravimetric analyses coupled with differential scanning calorimetry to investigate what happened to the composite upon heating.
They discovered that the ionic liquid began decomposing upon heating, and the partially decomposed ionic liquid fragments helped stabilize the rapidly decomposing ZIF-8 organic ligands. This stabilization kept the material from decomposing too soon and allowed the material to reach the decreased melting temperature—and successfully undergo the process to become a glass.
The researchers were excited to discover that the ZIF-8 melt-quenched glass had a glass-forming ability exceeding those of previously reported superstrong glasses from conventionally meltable ZIFs.
“It offers exciting opportunities to melt other nonmeltable crystalline MOFs, potentially enabling a broad range of hybrid glasses with a variety of physicochemical properties and corresponding applications, in particular, ones which are derived from MOF architectures with large pore sizes,” they conclude.
In an email, senior author and University of Jena professor Lothar Wondraczek says the team is now looking into how this approach can be applied to other traditionally unmeltable MOFs.
The open-access paper, published in Nature Communications, is “Ionic liquid facilitated melting of the metal-organic framework ZIF-8” (DOI: 10.1038/s41467-021-25970-0).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Glass


