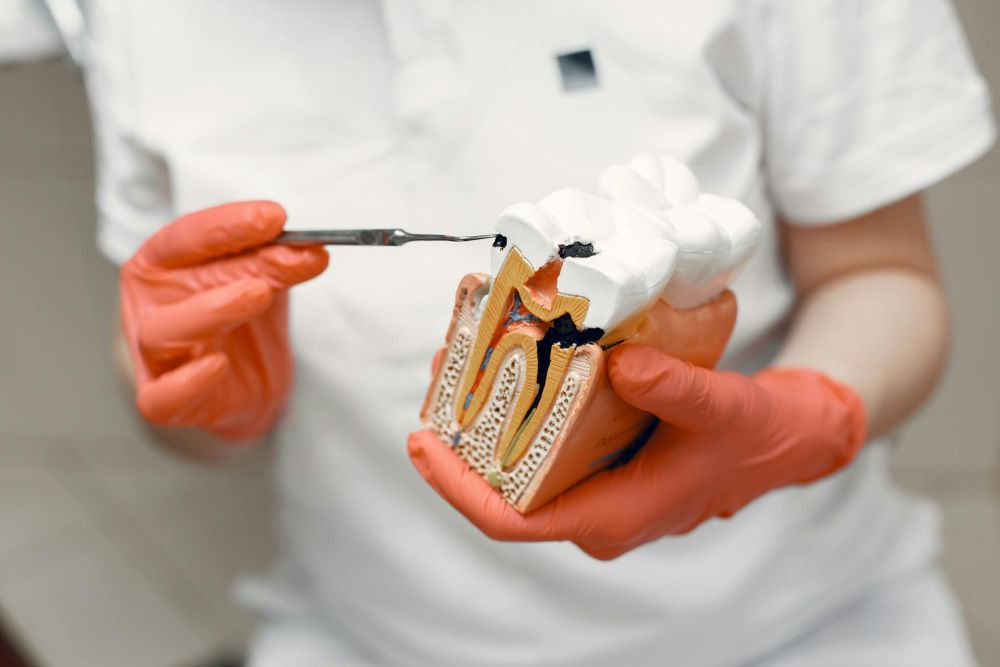
[Image above] Giant cell tumors of bone are aggressive, semimalignant tumors that can grow quickly and destroy bone close to a joint, such as the knee. Credit: Joerg, Flickr (CC BY 2.0)
A few months ago, one of my aunts had a tumor biopsied to determine if it was cancerous. Thankfully, it was benign, which was a huge relief to her and everyone in the family.
Even though it wasn’t cancerous, the doctors went ahead and removed the growth because benign tumors can still be dangerous—they can put pressure on critical organs or block the pathway of important nutrients as they grow.
Giant cell tumor of bone (GCTB) is one example of a noncancerous but aggressive tumor. These semimalignant tumors often occur in younger adults (20–40 years old) and typically grow at the ends of the body’s long bones, such as at the lower end of the femur (thighbone) or upper end of the tibia (shinbone), close to the knee joint.
GCTBs are considered aggressive because they can grow quickly and destroy bone close to a joint. Though the cause of these tumors is unknown, researchers do know that GCTBs are composed of three different cell types:
- Neoplastic (abnormally growing) stromal cells, or cells that makes up certain types of connective tissue;
- Osteoclast-like multinucleated giant cells, or cells that absorb bone tissue during growth and healing; and
- Macrophage-like giant cell precursors, or cells that use their plasma membrane to engulf other cells or particles.

Illustration of where giant cell tumors of bone most commonly form in the human body. Credit: Frank Gaillard, Wikimedia (CC BY-SA 3.0)
Standard treatment for GCTBs involves intralesional resection, a procedure in which the tumor is entered and then removed piecemeal from the body. However, this procedure can leave behind microscopic and macroscopic amounts of tumor in the surrounding tissues. To minimize risk of recurrence, a comparably wide resection including healthy surrounding bone tissue is necessary—which can generate further large bone defects.
There are several approaches for treating bone defects resulting from tumor resection, the most common being to fill the defect with the biologically inactive material polymethyl methacrylate (PMMA). However, considering the young age of most GCTB patients, a biological reconstruction is preferable because PMMA will remain in the bone as an inert material for the patient’s life and could cause some further problems, such as stress shielding at the PMMA/bone interface. Unfortunately, studies using cancellous bone as a biological grafting material show it is associated with much higher local tumor recurrence compared to PMMA.
“Therefore, when considering biologically active reconstruction aiming for consolidation of the bone after GCTB resection, the therapy option must commit to both, the lowest possible recurrence rate and the best possible osteogenic properties at the same time,” researchers write in a recent paper.
The researchers come from Heidelberg University Hospital and University of Erlangen-Nuremberg in Germany. The collaboration is led by Fabian Westhauser and Jörg Fellenberg at the Center for Orthopedics, Trauma Surgery, and Spinal Cord Injury at Heidelberg University Hospital, and Aldo R. Boccaccini, ACerS Fellow and head of the Institute of Biomaterials at University of Erlangen-Nuremberg.
In their paper, they hypothesize that bioactive glass may be a good option for treating the bone defects resulting from GCTB.
Bioactive glasses are a group of surface reactive inorganic biomaterials that were originally discovered in the late 1960s by Larry Hench. Since then, products based on the original bioactive glass formulation—Bioglass 45S5, a calcium sodium phosphosilicate—have been successfully implanted in millions of patients worldwide, mainly to repair bone and dental defects.
Researchers have extensively described the effects of bioactive glass on several bone precursor cells, such as bone marrow derived mesenchymal stromal cells. However, the interaction of bioactive glass with neoplastic stromal cells—a major component of GCTBs—is unknown.
“Due to the known differences of neoplastic and normal cells concerning energy metabolism and pH-tolerance and the important role of ion channels in driving malignant cancer cell behavior, we hypothesized that neoplastic [stromal cells] might react differently, ideally more sensitive against [bioactive glass] treatment,” the researchers write.
To test this hypothesis, they created bioactive glasses with five different compositions, including the traditional 45S5 formulation. They obtained tissue samples from patients who underwent surgery at Heidelberg University Hospital and gave informed consent to be included in the study prior to tissue sampling.
Remarkably, after a total observation period of 21 days, all analyzed bioactive glasses induced a significant decrease of neoplastic stromal cell viability, while the regular mesenchymal stromal cell viability was not affected and sometimes even improved after an initial decrease.
In particular, the traditional Bioglass 45S5 showed the most significant negative effects on neoplastic stromal cell viability, while the zinc-infused bioactive glass showed the most positive effect on mesenchymal stromal cell viability.
Currently, the precise underlying molecular mechanisms driving the bioactive glass and GCTB interaction is unknown. Boccaccini says in an email that the team is in the process of understanding the in vitro interaction better, including differences in behavior of the tumor cells compared to mesenchymal stromal cells.
“In the future, we are planning a close collaboration with the colleagues at Heidelberg University Hospital to design an in vivo model for GCTBs to explore interactions with bioactive glasses,” he says.
This study was funded by a grant from German Cancer Aid (Deutsche Krebshilfe).
The paper, published in Biomaterials, is “Selective and caspase-independent cytotoxicity of bioactive glasses towards giant cell tumor of bone derived neoplastic stromal cells but not to bone marrow derived stromal cells” (DOI: 10.1016/j.biomaterials.2021.120977).
Author
Lisa McDonald
CTT Categories
- Biomaterials & Medical
- Glass