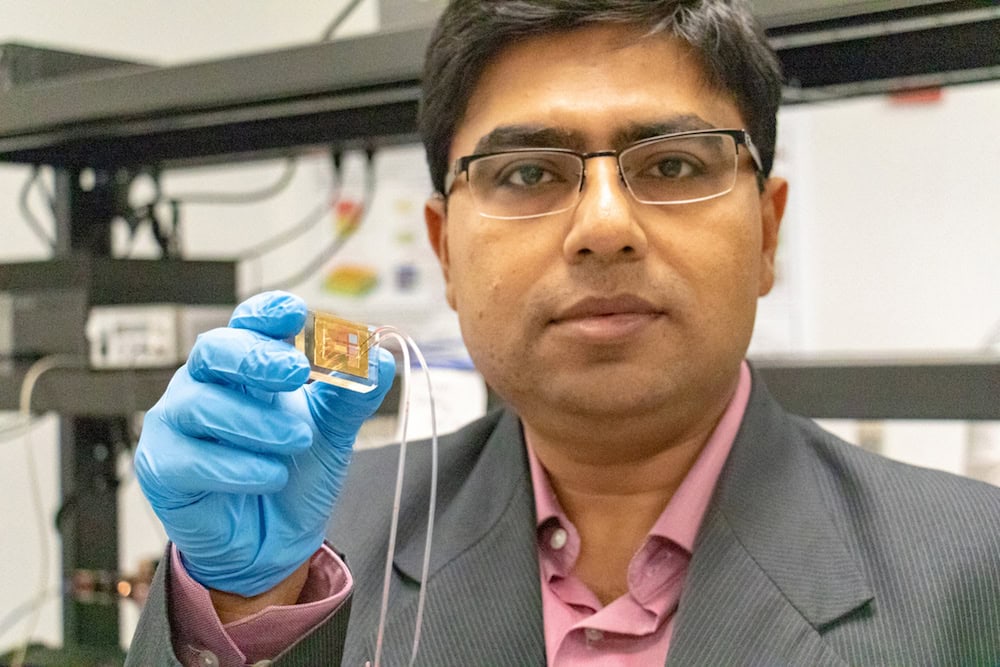
[Image above] Debashis Chanda, principal investigator and associate professor of physics at the University of Central Florida, holds the first-ever rapid detector of dopamine, created by researchers at UCF. Credit: University of Central Florida
How does the brain work?
That is a question pondered by children and world-renowned neuroscientists alike. And while you might satisfy a child’s interest by talking about how neurons pass information through the nervous system, neuroscientists know there is still a long way to go to fully understanding what exactly goes on in the brain.
One thing neuroscientists do know, however, is that neurotransmitters are an essential part of the process to pass information between neurons.
Neurotransmitters are chemicals released at the ends of a neuron that serve as a communication signal from one neuron to another. There are many different kinds of neurotransmitters, and one of those—dopamine—plays an important role in several central neurological processes (e.g., happiness, pleasure, cognition, fine motor control). On the flip side, lack of dopamine plays a role in several neurological disorders (Parkinson’s disease, depression, schizophrenia), while too much dopamine can be an indication of cancer.
By measuring dopamine levels in a person’s body, doctors are able to better diagnose and manage neurological disorders and diseases. Unfortunately, current technologies to measure dopamine concentration require rigorous sample preparation and specialized laboratory equipment, preventing these methods from being used in places lacking access to high-end technologies.
Developing point-of-care diagnostics for use in low resource settings is an important way to extend healthcare access to those most in need. And while doctors are ideally situated to know what technologies should be developed, materials scientists hold the key to turning desired designs into reality.
In the case of dopamine concentration point-of-care diagnostics, reality may be closer than ever thanks to researchers at the University of Central Florida. In a recent study, they describe how they created the first-ever rapid detector of dopamine. Their device takes the usual time-consuming, rigorous dopamine-detecting process and reduces it to just a few drops of blood and a single, palm-sized chip.

A schematic showing the integrated, dopamine-detector device created by University of Central Florida researchers. The detector is composed of a plasmonic sensor coupled to a microfluidic chip containing the plasma separator module. Credit: Reprinted with permission from Nano Letters, 2019, 19(1), pp. 449-454. Copyright 2019 American Chemical Society.
“There is no preprocessing needed,” says Abraham Vázquez-Guardado, a graduate of UCF and now a postdoctoral fellow at Northwestern University, in a UCF press release. “Our plan was to make a much quicker, enzyme-free kind of detection.”
The success of their method rests on integration of three essential elements: a sensitive nanostructured plasmonic substrate (NPS), selective cerium oxide nanoparticles (CNP), and a microfluidic plasma separator.
“The inorganic redox active CNP’s surface acts as selective [dopamine] binding sites for the selective optical detection on the NPS,” the researchers explain in the paper. “When coupled to the microfluidic system, the proposed device extracts blood plasma directly from the inlet bloodstream without additional sample preparation or purification and allows optical readout on the CNP-coated NPS.”
In other words, blood is separated into plasma by the microfluidic plasma separator—from there, cerium oxide nanoparticles capture dopamine molecules from the plasma. Captured dopamine changes how light is reflected from the sensor, creating an optical readout that indicates dopamine levels.
Of these three parts, Sudipta Seal, ACerS member and engineering professor and chair of UCF’s Department of Materials Science and Engineering, says use of cerium oxide nanoparticles was a key to the success.
“[Compared to other ceramics], nanoceria has the highest redox capability with switching +3/+4 states in a cell microenvironment. Therefore, it has an excellent electrochemical activity toward the oxidation of dopamine,” Seal explains in an email. “Also, increased concentration of oxygen vacancies in nanoceria leads to faster charge transfer.”
He adds that if other ceramics are created with increased concentrations of oxygen vacancies, they may exhibit similar activity, although this would need to be explored.
Although the chip allows for dopamine detection without rigorous preparation of blood, the researchers note this can result in protein fouling that must be dealt with during analysis.
“Performing the detection of [dopamine], as well as other antigen or biomarkers, in plasma without preparation or purification is susceptible to inherent protein fouling from the high protein content in biological fluids,” the researchers say in the paper. “Hence, further work is still needed to establish a generalized detection protocol.”
Seal says research is ongoing to examine this issue and other questions.
This study is not the first time Seal and his colleagues have used cerium oxide in health research. Check out their study from last year using cerium oxide to reduce cell damage from radiation exposure!
The paper, published in Nano Letters, is “Enzyme-free plasmonic biosensor for direct detection of neurotransmitter dopamine from whole blood” (DOI: 10.1021/acs.nanolett.8b04253).
Author
Lisa McDonald
CTT Categories
- Biomaterials & Medical
- Nanomaterials


