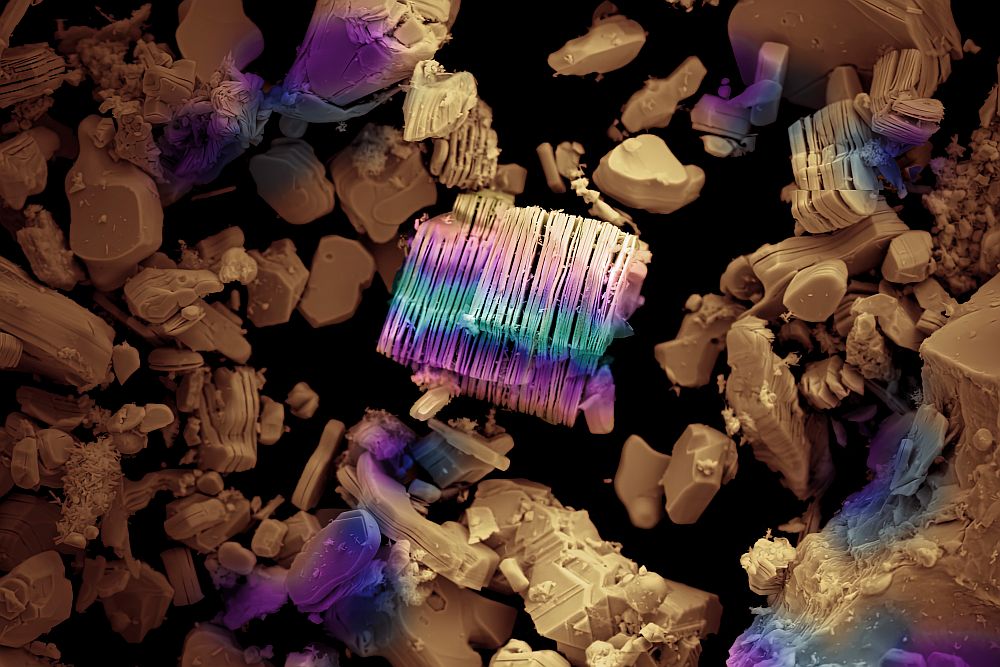
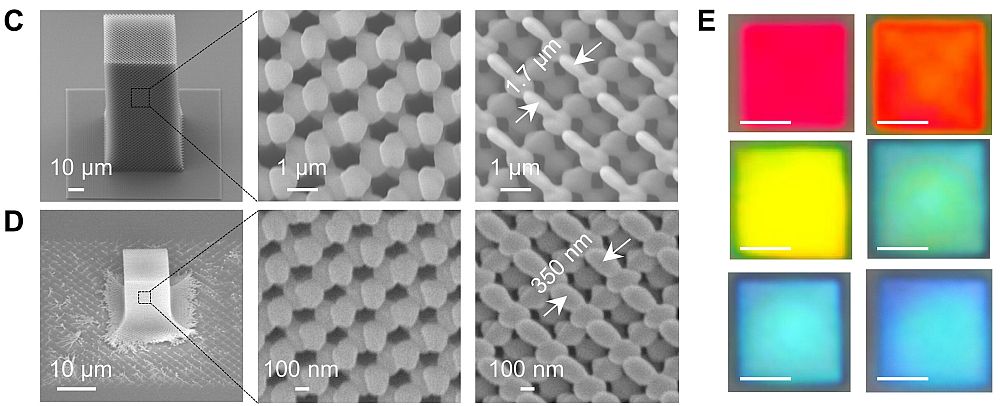
Stanford University researchers have discovered a new way to ‘decorate’ nanowires with coatings of metal oxide and noble metal nanoparticles that greatly improve surface area.
Check ’em out:
High-strength silk protein scaffolds for bone repair
(PNAS) Biomaterials for bone tissue regeneration represent a major focus of orthopedic research. However, only a handful of polymeric biomaterials are utilized today because of their failure to address critical issues like compressive strength for load-bearing bone grafts. In this study development of a high compressive strength (~13 MPa hydrated state) polymeric bone composite materials is reported, based on silk protein-protein interfacial bonding. Micron-sized silk fibers (10-600 µm) obtained utilizing alkali hydrolysis were used as reinforcement in a compact fiber composite with tunable compressive strength, surface roughness, and porosity based on the fiber length included. A combination of surface roughness, porosity, and scaffold stiffness favored human bone marrow-derived mesenchymal stem cell differentiation toward bone-like tissue in vitro based on biochemical and gene expression for bone markers. Further, minimal in vivo immunomodulatory responses suggested compatibility of the fabricated silk-fiber-reinforced composite matrices for bone engineering applications.
Prominent electrochromism through vacancy-order melting in a complex oxide
(Nature Communications) Electrochromes are materials that have the ability to reversibly change from one colour state to another with the application of an electric field. Electrochromic colouration efficiency is typically large in organic materials that are not very stable chemically. Here we show that inorganic Bi0.9Ca0.1FeO3-0.05 thin films exhibit a prominent electrochromic effect arising from an intrinsic mechanism due to the melting of oxygen-vacancy ordering and the associated redistribution of carriers. We use a combination of optical characterization techniques in conjunction with high-resolution transmission electron microscopy and first-principles theory. The absorption change and colouration efficiency at the band edge (blue-cyan region) are 4.8×106 m-1 and 190 cm2 C-1, respectively, which are the highest reported values for inorganic electrochromes, even exceeding values of some organic materials.
Light touch keeps a grip on delicate nanoparticles
(NIST Tech Beat) Using a refined technique for trapping and manipulating nanoparticles, researchers at the National Institute of Standards and Technology have extended the trapped particles’ useful life more than tenfold.* This new approach, which one researcher likens to “attracting moths,” promises to give experimenters the trapping time they need to build nanoscale structures and may open the way to working with nanoparticles inside biological cells without damaging the cells with intense laser light. NIST researchers’ new approach uses a control and feedback system that nudges the nanoparticle only when needed, lowering the average intensity of the beam and increasing the lifetime of the nanoparticle while reducing its tendency to wander.
New method increases the surface area of nanowires by “decorating” them with sinuous chains of metal oxide or noble metal nanoparticles
Though science has known for some time that ornamentation can greatly increase the surface area and alter the surface chemistry of nanowires, engineers at Stanford University have found a more effective method of decorating them that is simpler and faster than previous techniques. The development, say the researchers, might someday lead to better lithium-ion batteries, more efficient thin-film solar cells and improved catalysts that yield new synthetic fuels. The key to the Stanford team’s discovery was a flame. Engineers had long known that nanoparticles could be adhered to nanowires to increase surface area, but the methods for creating them were not very effective in forming the much-desired porous nanoparticle chain structures. Those other methods proved too slow and resulted in a too-dense, thick layer of nanoparticles coating the wires, doing little to increase the surface area. They dipped the nanowires in a solvent-based gel of metal and salt, then air-dried them before applying the flame. In the process, the solvent burns in a few seconds, allowing the all-important nanoparticles to crystalize into branch-like structures fanning out from the nanowires.
Materials Genome and the energy efficient soldier: University of Utah-led group gets $15 million from Army to help design new materials
US soldiers are increasingly weighed down by batteries to power weapons, detection devices and communications equipment. So the Army Research Laboratory has awarded a University of Utah-led consortium almost $15 million to use computer simulations to help design materials for lighter-weight, energy efficient devices and batteries. The consortium includes Boston University, Rensselaer Polytechnic Institute, Pennsylvania State University, Harvard University, Brown University, the University of California, Davis, and the Polytechnic University of Turin, Italy. The Utah-led consortium calls itself Alliance for Computationally-guided Design of Energy Efficient Electronic Materials. The Army says its grant to Utah is for Multiscale Multidisciplinary Modeling of Electronic Materials. “Designing new, transformational materials for our soldiers is the aim of our Enterprise for Multiscale Research of Materials,” says John M. Miller, director of the U.S. Army Research Laboratory. He says a strong foundation for that enterprise will be provided both by the University of Utah-led project, and by a related project led by Johns Hopkins University to understand how materials behave when subjected to high-velocity impacts – work aimed at developing new, lightweight materials to protect U.S. soldiers and vehicles. Miller says funding the research “also shows the Army’s commitment to the national Materials Genome Initiative.” President Barack Obama announced the initiative in June 2011 as a way to speed development and use of new materials.
Fraunhofer Institute for Ceramic Technologies and Systems demonstrates power without the cord
Because of the limited lifespan, battery power is not a feasible option for many applications in the fields of medicine or test engineering, such as implants or probes. Investigators in Germany have now developed a process that supplies these systems with power and without the power cord. Researchers at the Fraunhofer Institute for Ceramic Technologies and Systems IKTS succeeded in wirelessly transmitting power from a portable transmitter module to a mobile generator module – the receiver. “The cylindrical shaped transfer module is so small and compact that it can be attached to a belt,” says Holger Lausch, scientist at IKTS. The transmitter provides an electric current of over 100 milliwatts and has a range of about 50 centimeters. As a result, the receiver can be placed almost anywhere in the body. “With our portable device, we can remotely supply power to implants, medication dosing systems and other medical applications without touching them – such as ingestible endoscopic capsules that migrate through the gastrointestinal tract and transmit images of the body‘s inside to the outside,” says Lausch. The generator module can be traced any time – regardless of power transfer – with respect to its position and location. So if the generator is located inside a video endoscopy capsule, the images produced can be assigned to specifi c intestinal regions. If it is placed inside a dosing capsule, then the active ingredient in the medication can be released in a targeted manner.
Atomic-scale visualization of electrons confirms theory of iron-based superconductors
Research at Cornell University has for the first time confirmed key theoretical predictions about how iron-based high-temperature superconductors behave. J.C. Séamus Davis, the James Gilbert White Distinguished Professor in the Physical Sciences at Cornell and director of the Center for Emergent Superconductivity at Brookhaven National Laboratory, and colleagues report in the May 4 online edition of the journal Science that they have identified gaps in the energy levels of electrons in an iron-based superconductor that were predicted by leading theories in this new field. The gaps represent electrons that have paired up with twins from adjacent atoms to form so-called “Cooper pairs” that move through the conductor without interference. The research also confirms a prediction that the energy binding the Cooper pairs varies with the direction they take when leaving an atom. Studying crystals of a compound of lithium, iron and arsenic, LiFeAs for short, that becomes a superconductor at 15K (Kelvins, or Celsius degrees above absolute zero), the Cornell researchers found three of the five possible electron bands. “There are two more pairing gaps that we should have been able to detect, and we don’t know yet why not,” Davis said. But finding these three along with the directionality is enough to strongly support the theory, he said, and the measurements give the theorists numbers to plug in to refine and extend their predictions.
CTT Categories
- Basic Science
- Biomaterials & Medical
- Electronics
- Energy
- Glass
- Material Innovations
- Modeling & Simulation
- Nanomaterials