[Image above] Credit: PxHere
Biomimetics is the scientific research and development of human-made materials, parts, and products that emulate naturally occurring biological systems. From paper to ornate ironwork to plastic lumber, researchers have worked to replace natural products with biomimetic products for millennia. However, not all natural products are so easy to emulate.
Consider fingernails. They have an optimal combination of strength, flexibility, and structure to protect the nail bed from damage and disease. In addition, fingernails can be sharpened into weapons and scrapers. Though man has created artificial fingernails, they are fraught with issues including lack of “breathability,” or, in the language of materials science, porosity.
Thus, to achieve the ultimate goal of biomimetic research—the cost-effective manufacturing of products—researchers will sometimes template directly from natural materials to aid in emulating the unique structure and/or performance.
In a recent International Journal of Applied Ceramic Technology paper, researchers from Jilin Jianzhu University in China used rice hulls as templates to create a titanium dioxide (TiO2) photocatalyst for degrading microcystin, a harmful hepatic toxin in water ecosystems. Though rice hulls have no catalytic function, the structure provides a good combination of porosity and stability.
The authors compared five variations of their composite: unstructured nanoscale TiO2, structured TiO2, as-synthesized bismuth tungstate (Bi2WO6), and bismuth tungstate composites with the unstructured and structured TiO2. Of note, the composite with structured TiO2 has substantially higher surface area than with the unstructured by 100 m2/g (137 m2/g versus 37 m2/g).
In their research, the authors found that the structured composite material substantially outperformed all other variations with the highest amount of microorganism removal. Included in this article is exploration of the mechanism for the photocatalytic removal.
The paper, published in International Journal of Applied Ceramic Technology, is “The synthesis of biotemplate-based Bi2WO6/TiO2 composite photocatalyst for degradation of microcystin” (DOI: 10.1111/ijac.13830).
In a second ACT paper, authors from the Institute of Space Technology Islamabad in Pakistan focus on mimicking the function of biological systems using existing low-cost ceramics processing techniques to enhance the biological response of metallic implants.
Orthopedic implants such as hip and knee replacements are typically made from metals, including stainless steel and titanium. These materials have excellent mechanical properties for load-bearing joints and are mostly inert to bodily fluids. The implanted parts are designed so that bone grows into their external structures to lock them in place.
Unfortunately, these metals can induce foreign body reactions, with the resulting infections loosening the implant. When loosening occurs, additional surgeries are required.
Coatings are one method being explored to reduce the need for revision surgery. The ACT article describes the characteristics of optimal coatings, including seemingly contradictory requirements for antibacterial activity while increasing cellular binding. In other words, the coating must destroy foreign cells while promoting the growth of the individual’s own tissue.
In this paper, the authors prepare coatings using electrophoretic deposition, which is akin to electroplating of metals. An electric field (voltage) is applied across a bath containing charged coating mixtures, which deposit electrochemically on the electrodes—the same material as the metal implant in this case.
They optimized the composition of the coating, along with the deposition parameters of voltage and time. Parameters measured included deposition amount and consistency of the deposition process. Though no single set of conditions stood out as “the best,” the authors chose the best conditions based upon coating morphology.
The optimized coatings were tested for adhesion, structure, bone mineralization, and antibacterial properties, with very promising results.
The paper, published in International Journal of Applied Ceramic Technology, is “Ag–Sr doped mesoporous bioactive glass nanoparticles loaded chitosan/gelatin coating for orthopedic implants” (DOI: 10.1111/ijac.13702).
One of the most complicated examples for biomimetic development is bone replacement. When bones are damaged or diseased beyond their ability to self-repair, current therapies include metallic rods and pins or amputation, which can be painful. In contrast, bone replacement seeks to find materials that will temporarily hold bones together while the body’s natural bone and blood-vessel replacement processes can form permanent structures.
Bone replacement materials must be antibacterial while promoting osteogenesis (bone building), as is necessary for the coatings in the previous paper. In addition, they must have sufficient strength while retaining the structure and chemistry that enables the bone and blood-vessel building processes to create living tissue. And they must be absorbable by the body, but at the rate that is matched to the rate of tissue building. Fulfilling all these factors is not an easy challenge.
In a third ACT paper, researchers from the National Institute of Genetic Engineering and Biotechnology in Iran synthesized beta tricalcium phosphate as a bone growth promoter.
They mixed the phosphate with caprolactone, which is a common polymeric matrix for bone replacement therapies in different ratios. A key part of their sample preparation was freeze-drying of the fabricated composites to remove the organic solvents.
They tested the samples for structure, mechanical properties, mineralization, and bone cell attachment, growth, and viability. The live cell testing provides insight into the probability of success of these materials as bone scaffold candidates, and makes this article stand out from the others of this post.
The authors found that adding the phosphate improved all properties compared to the caprolactone, though no one set of conditions stood out as optimal. Nonetheless, their research shows promise for a low-cost method for fabricating items for this very complicated application.
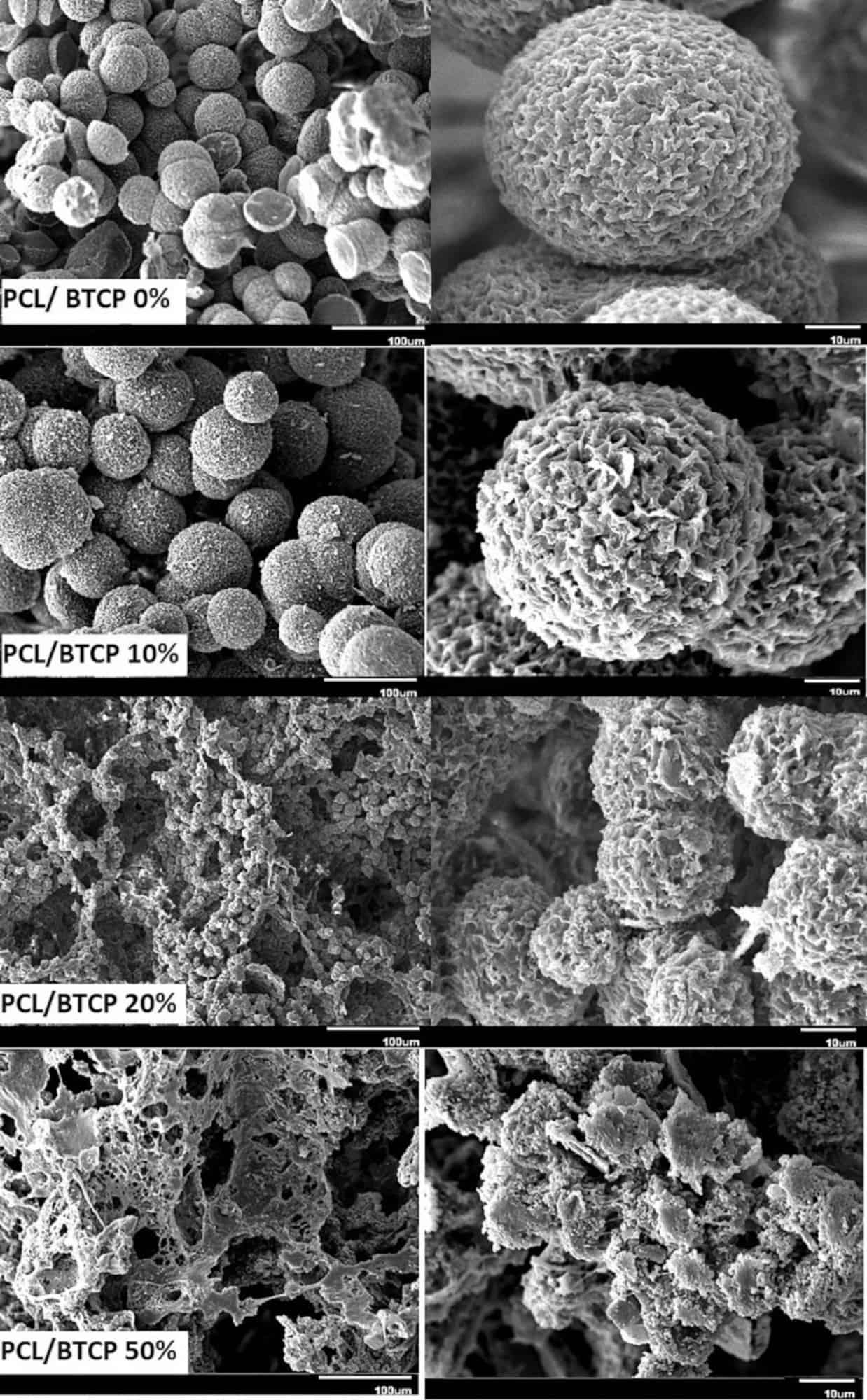
SEM micrographs showing the microstructure of the phosphate composite with different levels of the ceramic additive. Credit: ShiraliPour et al., International Journal of Applied Ceramic Technology
The paper, published in International Journal of Applied Ceramic Technology, is “Three-dimensional porous poly(ε-caprolactone)/beta-tricalcium phosphate microsphere-aggregated scaffold for bone tissue engineering” (DOI: 10.1111/ijac.13770).
Author
Jonathon Foreman
CTT Categories
- Biomaterials & Medical