[Image above] Common teeth whitening methods can damage teeth—could a proposed piezocatalysis effect-based method be safer? Credit: Wang et al., Nature Communications (CC BY 4.0)
A core ethos that shapes culture in the United States is the American Dream, which says every U.S. citizen has equal opportunity to realize economic and social success. However, research on this ethos in recent years reveals it is likely more “dream” than reality, as factors including race, family socioeconomic status, and early education greatly affect an individual’s ability to progress up the social and economic ranks.
One very visible symbol of the American Dream is teeth. Straight, white teeth are a bit of an obsession for Americans. But achieving such teeth can be very costly, meaning it can be difficult, if not impossible, for poor and working-class people to realize the “Hollywood” smile.
For people who have the economic resources to pursue pearly white teeth, they face significant cost in another way—potential damage to their teeth.
Common methods for whitening teeth—including professional cleaning and polishing, coverage with crowns or veneers, daily brushing with abrasive toothpaste, and bleaching—all can damage teeth.
“Both professional procedural cleaning and coverings require grinding or other enamel-cutting steps, which cause irreversible damage,” researchers write in a recent paper. Also, “abrasive cleaning exhibits limited efficacy and, furthermore, causes slight scratches to the surface of teeth.”
While bleach treatment is highly efficient, “bleaching with hydrogen peroxide may cause serious side effects, i.e., loss of organic matrix and increase of enamel micro-roughness,” they add.
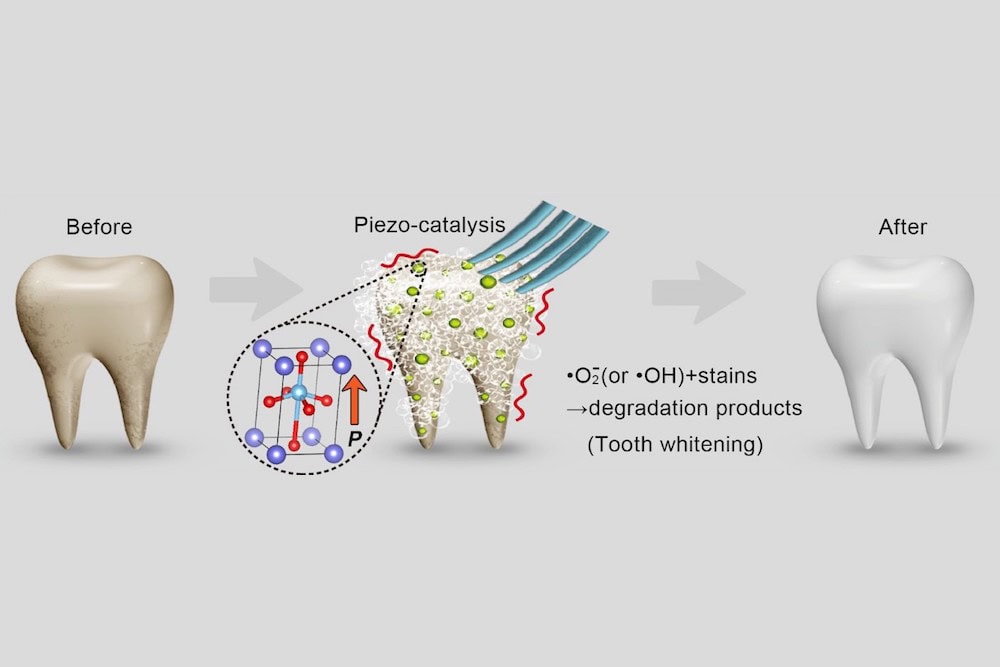
The researchers come from several universities in China, including Nanjing University of Science and Technology, Peking University School and Hospital of Stomatology, and Shanghai Normal University. And in their paper, they propose a new nondestructive method for whitening teeth.
Their method uses a mechanism similar to the one in bleaching. In bleaching, hydrogen peroxide releases unstable, reactive oxygen species during decomposition into water. These species attack organic pigment molecules on the surface of teeth and degrade staining compounds by oxidation.
“This mechanism suggests that a material with the capability to excite and release reactive oxygen species could be effective as a tooth whitening agent,” the researchers write.
A 2018 study explored producing reactive oxygen species using the photocatalysis effect of blue-light-activated TiO2 nanoparticles. While this method was nondestructive to teeth, it “may cause various photo-toxic and photo-allergic reactions, and in turn lead to damage to oral tissue, because blue light is required as a stimulus to produce reactive oxygen species,” the researchers explain.
In their new study, the researchers in China looked to produce reactive oxygen species using the piezocatalysis effect instead. In contrast to photocatalytic reactions, which are induced using light, piezocatalytic reactions are mechanically induced.
The researchers explain that in application, piezoelectric nanoparticles would replace nanosized abrasive particles in toothpaste; mechanical vibrations caused by normal tooth brushing would trigger the piezocatalytic reaction. For this study, though, the researchers created liquids of piezoelectric nanoparticles and used ultrasonic vibration to simulate daily tooth brushing.
For the piezoelectric nanoparticles, they used barium titanate (BaTiO3, BTO), a well-known piezoelectric material. They used both poled and unpoled BTO since “piezoelectric properties can be significantly improved by electric poling,” and they wanted to investigate the difference.
The researchers stained teeth with either black tea, blueberry juice, wine, or a combination of these liquids. They then placed the teeth in either water or (un)poled BTO liquids and vibrated the samples up to 10 hours.

Photographs of teeth under treatment using ultrasonic vibration in (top) pure deionized water and (bottom) turbid liquid of poled BTO nanoparticles for 0, 1, 3, and 10 hours, respectively. Credit: Wang et al., Nature Communications (CC BY 4.0)
The poled BTO liquid noticeably whitened teeth after vibration for 3 hours. In contrast, the unpoled BTO liquid and water showed negligible whitening effect.
To further verify these results, the researchers then carried out an experiment using a lab-made electric toothbrush setup. The toothbrush cleaned teeth by vibrating water or poled BTO liquid periodically at 2-minute intervals for 10 hours. As with ultrasonic vibration, the poled BTO liquid showed a whitening effect while water did not.
While these results showed piezocatalytic reactions led to whitening, the researchers needed to verify the method was nondestructive to teeth.
To verify, they compared scanning electron microscopy images of teeth cleaned with either poled BTO or hydrogen peroxide. The results were as expected—the tooth whitened using hydrogen peroxide showed erosion and extensive damage in its enamel, while the BTO-whitened tooth did not.
“The relative lack of enamel deterioration using BTO nanoparticles further indicates that the reactive species created by piezo-catalysis of BTO nanoparticles are less than that created by 3% H2O2 during the tooth whitening process,” the researchers write.

Scanning electron microscope images of an identical tooth a) before whitening treatment, b) after piezocatalysis whitening in poled BTO turbid liquid for 3 hours, and c) after further whitening by 30% H2O2 for 2 hours. Scale bars are 100 μm (top) and 50 μm (bottom). Credit: Wang et al., Nature Communications (CC BY 4.0)
In the conclusion, the researchers discuss the potential this method holds for daily life.
“Unlike existing techniques, piezo-catalysis tooth whitening has the potential to be widely adopted for home use, without requiring significant investment from consumers in either time or resources,” they write.
In particular, the proposed method “can be effective when incorporated in a daily toothbrushing regime that replaces toothpaste made using traditional, passive abrasives with piezoelectric particles—particularly when used in conjunction with the strong, high frequency excitation present in traditional electric toothbrushes.”
The open-access paper, published in Nature Communications, is “Piezo-catalysis for nondestructive tooth whitening” (DOI: 10.1038/s41467-020-15015-3).
Author
Lisa McDonald
CTT Categories
- Biomaterials & Medical
- Material Innovations


