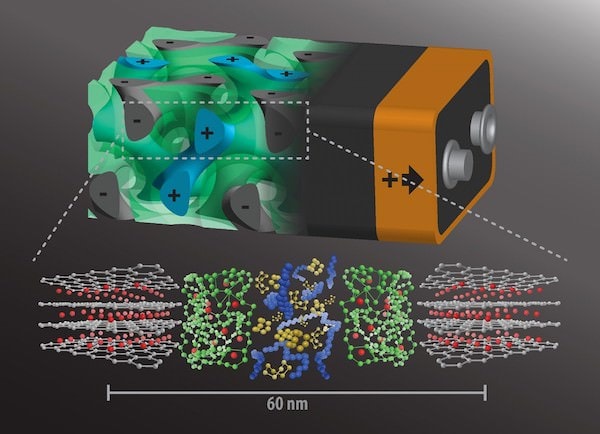
[Image above] A rendering of the 3-D battery architecture (top; not to scale) with interpenetrating anode (grey, with minus sign), separator (green), and cathode (blue, plus sign), each about 20 nanometers in size. Below are their respective molecular structures. Credit: Wiesner Group, Cornell University
If there’s one thing we’d like our electronic devices to do (that they don’t already do) is charge more quickly.
And batteries for said devices have been the subject of much research. Scientists have studied a variety of ways to make higher-performing batteries, including electroplating, adding sugar, using MXenes as electrode materials, and employing an asphalt derivative in anodes, among other research.
Typically, batteries have a positive and a negative terminal (cathode and anode) separated by an electrolyte, where electron transfer generates an electric current when the electrodes are connected into a circuit.
And ever since the early days, that’s how the average battery was constructed.
But researchers at Cornell University have a different idea about how to construct a more efficient battery. In a proof of concept, Spencer T. Olin Professor of Engineering in the Department of Materials Science and Engineering, Ulrich Wiesner, led a collaboration of researchers who designed a battery in a 3-D architecture at nanoscale. Their design intertwined the anode, cathode, and separator in a “self-assembling, 3-D gyroidal structure, with thousands of nanoscale pores filled with the components necessary for energy storage and delivery,” according to a Cornell news release.
“This three-dimensional architecture basically eliminates all losses from dead volume in your device,” Wiesner says in the release. “More importantly, shrinking the dimensions of these interpenetrated domains down to the nanoscale, as we did, gives you orders of magnitude higher power density. In other words, you can access the energy in much shorter times than what’s usually done with conventional battery architectures.”

Credit: Cornell University/Wiesner Group
The team’s concept uses self-assembly of block copolymers to develop the battery’s architecture. This included the battery’s anode, made of gyroidal thin carbon films, which featured “thousands of periodic pores on the order of 40 nanometers wide,” according to the news release. They coated the pores with a 10 nanometer-thick insulating separator via electropolymerization.
What’s significant is that the separator is free of tiny pinholes that could contribute to battery failure, such as a fire or explosion.
They used sulfur for the cathode material and backfilled with PEDOT, an electronically conducting polymer. To prove the concept, researchers addressed numerous challenges, especially degradation of the PEDOT charge collector that happens when the volume changes during discharging.
Wiesner says there were other challenges as well. “Another issue we encountered was that the current densities we were able to measure were on the low side,” he explains via email. “We need to find out what is potentially causing this: electronic contact to the ‘current collectors,’ especially the PEDOT, the conductivity of the PEDOT, the interfacial resistance of the lithium ions traveling from the carbon through the PPO to the sulfur—all of these could be potential causes.
“None of the interfaces were particularly engineered, but of course they are tremendously important since as the result of the interpenetrating nano-architecture, the device has VERY large interfaces,” he adds.
The researchers have already applied for a patent for their work. Meanwhile, they still continue to perfect their battery.
Wiesner is excited about the potential for their battery, after hearing from multiple experts over the years that such a battery was impossible to build.
“For more than 15 years, people in the battery community have been talking about such an approach to building a battery,” he adds in his email, “but to the best of our knowledge, no one has been able to achieve the interpenetrating architecture with all components having dimensions of order 20 nm.
“As we demonstrated, it can be done,” he says excitedly. “We now hope that others in the community will be inspired and help build on this early work in order to explore how far this can be pushed, and whether at the end this architecture will reach the full commercial scale.”
The paper, published in Energy and Environmental Science, is “Block copolymer derived 3-D interpenetrating multifunctional gyroidal nanohybrids for electrical energy storage” (DOI: 10.1039/C7EE03571C).
Did you find this article interesting? Subscribe to the Ceramic Tech Today newsletter to continue to read more articles about the latest news in the ceramic and glass industry! Visit this link to get started.
Author
Faye Oney
CTT Categories
- Basic Science
- Electronics
- Energy
- Material Innovations
- Modeling & Simulation
- Nanomaterials


