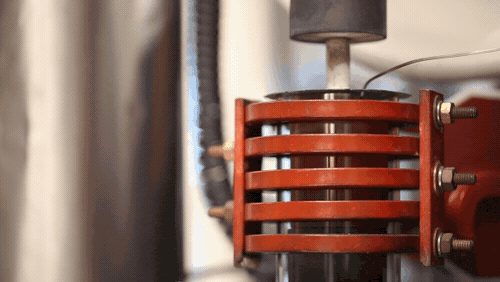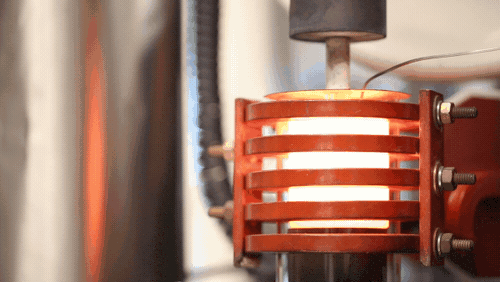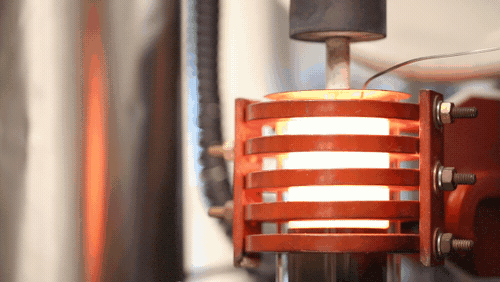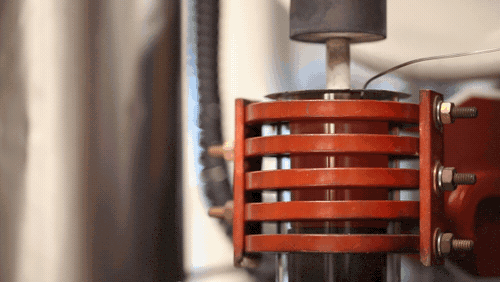
[Image above] A demonstration of a machine that uses heat to densify a ceramic known as LLZO at 1,225 degrees Celsius. Credit: Evan Dougherty, Michigan Engineering
There is a considerable amount of ongoing research to develop faster-charging and longer-lasting batteries, especially lithium batteries that power many of our devices.
Lithium-ion batteries, the portable power standard for nearly three decades, are used in a whole slew of devices, including smartphones, computers, cameras, watches, electronics, medical devices, and electric and plug-in hybrid electric vehicles.
So there are a number of reasons, including those mentioned above, for scientists to continue to improve upon the lithium battery.
One of the biggest challenges for researchers is preventing the formation of dendrites inside the electrolyte over time—caused by the battery’s continuous charge-discharge cycle. Over time, they can lead to combustion and possible fires.
The other issue with lithium-ion batteries is that the charge-discharge cycle causes them to degrade over time. But lithium has always been a superior material for batteries because of its high energy density and conductivity.
Researchers at the University of Michigan may have solved these problems with a new type of battery. Led by associate professor of mechanical engineering Jeff Sakamoto, they developed a solid-state lithium battery with a ceramic electrolyte that stabilizes the surface and “allows batteries to harness the benefits of lithium metal—energy density and high-conductivity—without the dangers of fires or degradation over time,” according to a University of Michigan news release.
“What we’ve come up with is a different approach—physically stabilizing the lithium metal surface with a ceramic,” Sakamoto says in the release. “It’s not combustible. We make it at over 1,800 degrees Fahrenheit in air. And there’s no liquid, which is what typically fuels the battery fires you see. You get rid of that fuel, you get rid of the combustion.”
In early tests of the battery at low charge, the lithium metal grew through the ceramic electrolyte and short-circuited the battery. So the researchers used chemical and mechanical treatments to “provide a pristine surface for lithium to plate evenly, effectively suppressing the formation of dendrites or filaments,” according to the release. The bonus is a dramatic improvement in charging rates, Sakamoto adds.
He explains that it typically takes 20–50 hours to fully charge a lithium metal car battery. With their battery, he says, they demonstrated a charging time in less than three hours.
Also, after conducting charge-discharge tests of the ceramic electrolyte over a period of 22 days, the team found no apparent degradation—a condition they claim other solid state battery electrolytes cannot match after repeated cycling. “We aren’t aware of any other bulk solid-state electrolyte performing this well for this long,” mechanical engineering postdoc fellow Nathan Taylor asserts.
Scaling the technology is the group’s next step in their research. “Within the upcoming year we will try to realize key aspects of our solid-state battery manufacturing vision,” Sakamoto explains in an email. “It involves the development of a new ceramic processing technique to fabricate of large area, thin film Li-ion conducting membranes. Currently, we are discussing pilot or pre-pilot line manufacturing with potential industrial partners.
“In our latest paper, we demonstrated a step increase in cycling performance of Li metal anodes protected by our ceramic technology,” he adds. “However, demonstrating the manufacturing of large area, thin film ceramic membranes and their integration with electrodes is the next major challenge.”
The paper, published in the Journal of Power Sources, is “Demonstration of high current densities and extended cycling in the garnet Li7La3Zr2O12solid electrolyte” (DOI: 10.1016/j.jpowsour.2018.06.055).
Want to read more articles like this? Subscribe to the Ceramic Tech Today newsletter to continue to receive the latest news in the ceramic and glass industry right in your inbox! Visit this link to get started.
Author
Faye Oney
CTT Categories
- Basic Science
- Electronics
- Energy
- Manufacturing
- Material Innovations
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026
Solid-state batteries turn heads at CES 2026
January 29, 2026


