
P. aeruginosa growth in the presence of ceria nanoparticles at three tested time points (1, 6, and 24 hours). The volume ratio of bacterial solution (107 bacteria/mL) and ceria nanoparticles is 5:1. Data is represented as mean ± standard deviation, n=3; *P< 0.01 compared with the tryptic soy broth (TSB) control group after 24 hours of treatment. **P < 0.05 compared with the TSB control group after 6 hours of treatment. Dex = dextran and PAA = polyacrylic acid. Credit: Wang et al., Dovepress.
Medically speaking, we live in marvelous times. Doctors prop heart arteries open with stents, replace bum knees and hips to liberate arthritics from wheelchairs, and implant new teeth when the originals betray us and decay beyond repair.
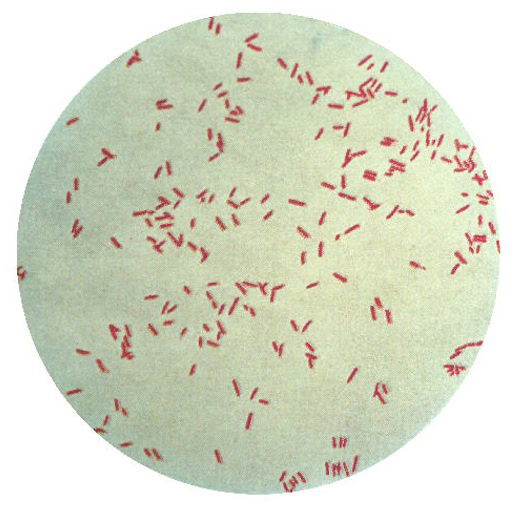
Despite the promise of relief, some anxiety over such “procedures” is normal—“you’re going to put that where?” Unfortunately, nasty stowaways sometimes hitch a ride on medical devices, and the bacterium known as Pseudomonas aeruginosa (shown in the gram stain image above; credit: US Centers for Disease Control) is among the most tenacious. Fighting off bacteria with antibiotics has proven challenging because the simple creatures are remarkably adaptable and can spawn generations of antibiotic-resistant descendants, so-called “superbugs.” P. aeruginosa further defends itself by secreting a biofilm, which makes a colony of it even more difficult to treat.
Besides the ongoing hunt for the next generation of antibiotics, pharmaceutical nanotechnology may open new avenues for fighting antibiotic-resistant bacteria, as this Nanowerk article explains. Nanostructured inorganic materials like titania, silver, and now ceria have properties that could be game-changing in the war between bugs and man, too. Ceria’s antioxidant properties have been known for at least five years, and a new paper published by a group led by Thomas Webster, professor at Northeastern University in Boston, Mass., reports on their study of the antibiotic properties of ceria nanoparticles as well as their approach to encapsulating ceria to avoid toxic effects. Webster’s graduate student and lead author, Qi Wang, condensed the article to its essentials for CTT readers (Credit: Dovepress; Creative Commons License). The full-text, open access paper is “Inhibited growth of Pseudomonas aeruginosa by dextran- and polyacrylic acid-coated ceria nanoparticles,” by Qi Wang, J. Manuel Perez, and Thomas J Webster, International Journal of Nanomedicine, 2013, No. 8, 3395–3399.
In the bacterial battle of good vs. evil, the “magic bullet” may be a nanostructured oxide.
Ceria nanoparticles for preventing bacterial growth
Condensed by Qi (Gavin) Wang
As a highly abundant material (66.5 ppm in the Earth’s crust), cerium oxide (CeO2) is technologically important due to its wide range of applications, for example in catalysts for the elimination of toxic automobile exhaust gases, oxygen sensors, fuel cells, electrochromic thin-films, and so on. Although ceria nanoparticles have been studied for numerous applications in traditional science and engineering, there have been almost no studies regarding the potential biomedical applications of ceria until recently, when researchers demonstrated that ceria nanoparticles possess antioxidant properties at physiological pH values. The ability of these nanoparticles to act as an antioxidant lies in their ability to reversibly switch from Ce3+ to Ce4+. In recent studies [published over the last seven years], this antioxidative ability enabled the application of ceria nanoparticles to protect against radiation damage, oxidative stress, and inflammation.
To build on these exciting medical applications of CeO2 nanoparticles, researchers reported for the first time the influence of ceria nanoparticles on the growth of bacteria, specifically Pseudomonas aeruginosa. P. aeruginosa is an aerobic gram-negative bacterium that is an important cause of various infections, especially in hospitals. Hospitalized patients may be colonized with P. aeruginosa on admission or during their hospital stay, and P. aeruginosa can be isolated from nearly any conceivable source within hospitals. In this study, two biocompatible molecules (dextran and polyacrylic acid) were used to coat ceria nanoparticles to enhance its stability in aqueous solutions and minimize potential toxicity. Figure 1 shows the results of bacterial studies. The growth of P. aeruginosa was strongly inhibited in the presence of dextran or polyacrylic acid coated ceria nanoparticles after 24 hours compared with no ceria controls (P < 0.01). The inhibition of P. aeruginosa growth reached 55.41% and 36.44% for dextran and polyacrylic acid coated ceria nanoparticles, respectively. Critically, all of this inhibition occurred without the use of antibiotics, only when using the ceria nanoparticles themselves.
Importantly, because ceria nanoparticles have been shown to suppress the generation of reacting oxygen species (ROS), its antibacterial properties demonstrated here for the first time could be different from many other nanoparticles (such as zinc oxide nanoparticles, silver nanoparticles, and so on) which are believed to kill bacteria mainly through the generation of ROS. Although requiring additional investigation, the possible mechanism for antibacterial action of ceria nanoparticles could be that the dextran and polyacrylic acid coatings or ceria nanoparticles themselves interact with specific proteins on bacterial cell membranes to alter the permeability of cell membranes, thus, killing bacterial cells. Further studies, such as longer treatment and elucidating the mechanism of action, are important to achieve a better understanding and application of ceria in numerous antibacterial applications.
Overall, the results of the reported study shows that ceria nanoparticles significantly inhibited the growth of P. aeruginosa, revealing a promising application of ceria nanoparticles, which could potentially serve as a novel antibacterial agent for various biomedical applications.
Author
Eileen De Guire
CTT Categories
- Biomaterials & Medical
- Nanomaterials