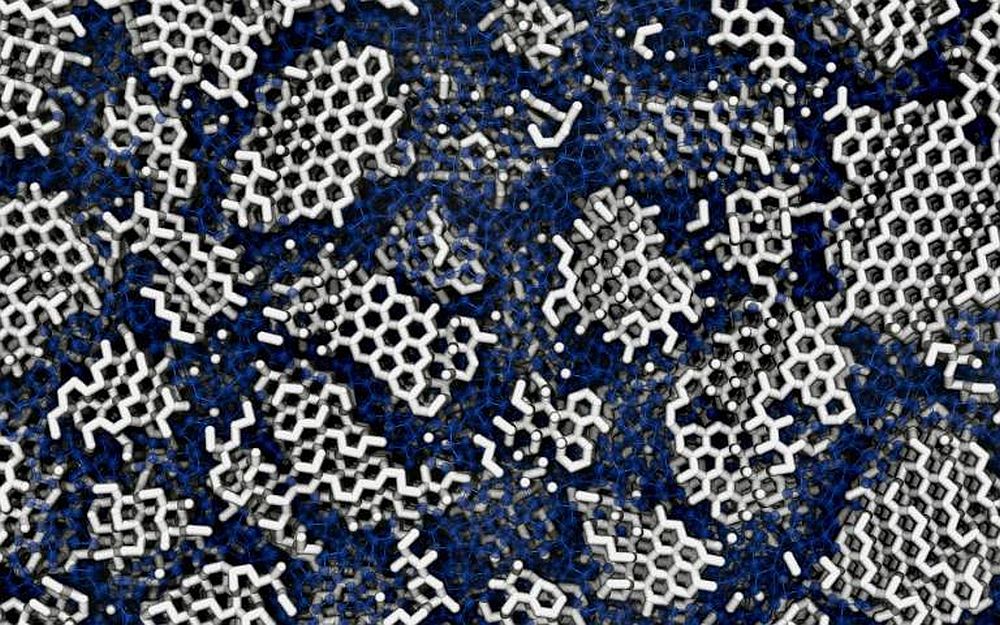
[Image above] Visual representation of the structure of low-density amorphous ice. Many tiny crystallites (white) are concealed in the amorphous material (blue). Credit: Michael B. Davies, University College London and University of Cambridge
As the Voyager 1 spacecraft was hurtling on its way to interstellar space in February 1990, it momentarily turned around to snap a “family portrait” of the planets in our solar system. This brief interlude during its journey, which occurred in large part due to U.S. astronomer and planetary scientist Carl Sagan, resulted in the famous Pale Blue Dot image, which captures Earth at a distance of 3.7 billion miles (6 billion kilometers) from the sun.
Although Earth was just a point of light about a pixel in size, the iconic blue color still managed to encapsulate what makes our planet so unique: a vast body of liquid water that covers approximately 71% of Earth’s surface. While liquid water may exist on other planets and moons, it is suspected to exist below the surface or trapped within tiny glass beads, as on our moon.
Yet there is another form of water that is incredibly common in the universe: water ice (not that water ice, Philly folks). This frozen version of H2O is found in comets, icy moons, and interstellar clouds, and unlike crystalline ice on Earth, it typically exists in a low-density amorphous (LDA) form.
Scientists have long believed that LDA is entirely amorphous, and this belief was a foundation for cosmochemistry, or the study of the chemical composition of the universe. For example, scientists believe that the amorphous nature of LDA makes it a safe vehicle for bringing delicate organic material through space and then through Earth’s atmosphere. (When I was researching clay as the catalyst for life on Earth, I read about the theory that amino acids traveled here in the ice of a comet.)
However, in the past couple of decades, some studies have pointed toward a more “crystal-like” nature than a purely amorphous state for LDA. Now, a recent open-access paper by researchers at the University College of London and Cambridge University appears to confirm this hypothesis.
Revealing the partially crystalline structure of LDA
The authors of the recent paper are part of the same research lab that first identified medium-density amorphous ice, which improved our understanding of liquid water.
For this work, they found that computer simulations of LDA best matched measurements from previous experiments if the ice contained tiny crystals (~3 nm) embedded within its disordered structures. This finding suggests a more complex thermal and pressure history for LDA than was previously thought.
The open-access paper, published in Physical Review B, is “Low-density amorphous ice contains crystalline ice grains.” (DOI: https://doi.org/10.1103/PhysRevB.112.024203).
Why crystals in LDA are a big deal for science
This finding could have major implications for cosmochemistry and astrophysics, as explained by first author Michael B. Davies, who did the work as part of his Ph.D. studies, in a press release.
“…ice is involved in many cosmological processes, for instance, in how planets form, how galaxies evolve, and how matter moves around the Universe,” Davies says. “We now have a good idea of what the most common form of ice in the Universe looks like at an atomic level.”
Amorphous ice also is a requirement for many areas of scientific research here on Earth, so the discovery that LDA is not completely amorphous means that adjustments may need to be made to local experimental processes.
Redefining cryopreservation and microscopy
The avoidance of ice crystallization in biological matter is key to successful cryopreservation. Meanwhile, in cryo-electron microscopy, completely amorphous LDA would help prevent sample damage and improve resolution. This discovery could encourage researchers in these fields to reevaluate their processes for producing amorphous ice and possibly adjust their methods.
Understanding the anomalies of water
The behavior of water is notoriously strange. For example, unlike most liquids that become denser as they cool, water reaches its maximum density at 4°C (39.2°F). Below this temperature, it expands and becomes less dense, which is why ice floats on liquid water.
Previous theories have suggested that water may be a mixture of both high-density and low-density phases depending on the arrangement of the molecules (the two-state theory). The new discovery of crystalline regions in LDA, in addition to the discovery of medium-density amorphous ice, calls that model into question and may change how we interpret the response of water to pressure and temperature.
The fact that LDA retains a memory of its formation pathway (i.e., its parent phase) and that this memory influences its recrystallization behavior is a critical finding. This behavior suggests that the initial preparation method is a crucial factor in determining the final properties of a material, even after subsequent transformations.
As our ability to study very small structures within materials improves, so does our understanding of the relationship between structures and functions. This discovery is a powerful reminder that our assumptions about even the most common substances may not be accurate.
Author
Becky Stewart
CTT Categories
- Aeronautics & Space
- Basic Science