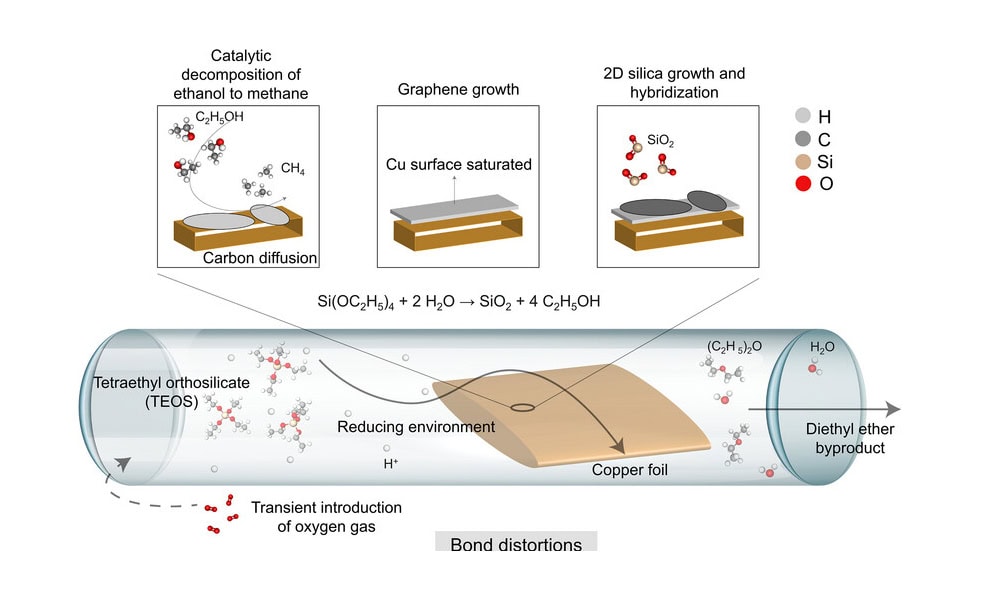
[Image above] Illustration of a one-pot, liquid precursor-mediated, low-pressure chemical vapor deposition process that creates genuine 2D hybrid materials consisting of graphene and silica glass. Credit: Iyengar et al., Advanced Materials (CC BY-NC-ND 4.0)
Ever since Andre Geim and Konstantin Novoselov received the 2010 Nobel Prize in Physics for their pioneering work in isolating and characterizing the 2D material graphene, interest in this material has grown significantly. According to the Graphene Council, more than 370,000 research papers and 150,000 patents have been published. Commercialization of graphene-based materials—including even car wax, reviewed in this video—now represents 250 plus companies.
While graphene by itself is impressive—demonstrating excellent strength and conductivity thanks to its hexagonal lattice of carbon atoms—many groups are now exploring the possibilities of combining graphene with other materials. However, almost universally, these experiments simply involve using graphene to enhance the properties of existing products or materials, not the creation of “new” materials with properties drastically different from its components.
This distinction is due to how 2D materials are traditionally layered together. As noted in a Rice University press release, “Most efforts simply stack these atom-thin sheets like a deck of cards, but the layers typically lack significant interaction between them.” While some layering methods, such as Janus configurations, do encourage more interaction between the different layers, this method is limited to materials within the same class, particularly transition metal dichalcogenides.
In a breakthrough for 2D materials processing, researchers led by Rice University created a genuine 2D hybrid material by chemically integrating graphene and silica glass—two fundamentally different 2D materials—into a single, stable compound called glaphene.
In glaphene, “The layers do not just rest on each other—electrons move and form new interactions and vibration states, giving rise to properties neither material has on its own,” explains first author Sathvik Ajay Iyengar, doctoral student at Rice University, in the press release.
Graphene is a semi-metal (zero-bandgap semiconductor) with long-range ordering, whereas silica glass is an insulator (bandgap ~ 9.0 eV) with short-range ordering. Glaphene, on the other hand, has an experimental bandgap of 3.6 eV, similar to the wide bandgap semiconductor gallium nitride (3.4 eV).
To create the novel 2D hybrid material, the Rice-led team developed a one-pot, liquid-precursor chemical vapor deposition process. Tetraethyl orthosilicate (TEOS) was chosen as the main precursor because it is a source of both carbon and silica. The hydrocarbon source thermally decomposes and catalytically forms methane and ethane gases, which drives graphene growth on copper substrates. TEOS also produces silica as one of its thermal decomposition products, but it can also form silica via hydrolysis.
The overall process, which is scalable to several centimeters, involves two steps:
- Graphene grows under low pressure (10-3 Torr) at elevated temperatures in an argon/hydrogen atmosphere.
- Oxygen is slowly introduced to promote water formation and thus silica growth via hydrolysis.
High-quality growth of graphene saturated the substrate before silica growth dominated. Further analysis showed the oxygen content played a role. If too little oxygen was present, amorphous silica formed on high-quality graphene. If too much oxygen was present, amorphous silica formed on amorphous carbon.
The authors used a variety of microscopy and spectroscopy methods to characterize the final material:
- Low-magnification transmission electron microscopy and energy-dispersive X-ray spectroscopy indicated layered ordering.
- The interlayer structure was chemically validated using optical and electron spectroscopy, mass spectrometry, and atomic-resolution electron microscopy.
- Spectroscopic analysis also showed that effective charge transfer resulted in interlayer hybridization, resulting in a structure that was more stable than the sum of its layers.
- Depth-dependent time-of-flight secondary-ion mass spectrometry indicated interlayer interaction between silicon in the silica glass layer and carbon in the graphene layer.
- Both Raman spectroscopy and X-ray photoelectron spectroscopy verified that no competing species formed during chemical vapor deposition growth.
- Qualitative stoichiometric X-ray photoelectron spectroscopy analysis indicated a close agreement between the theoretical model (SiO3C2) and experimental values (SiO2.1C2.1)
Based on these results, the authors proposed a hybrid metastable structure with a larger unit cell and a lower biaxial lattice mismatch (0.3%). This result is in contrast to a previous study that reported a 7% lattice mismatch between 2D silica glass on graphene. Further analysis predicted a metastable structure accommodates the lower strain by slight out-of-plane bending of silicon–oxygen bonds.
A unique feature of the hybrid glaphene was its disordered crystalline structure, which is not observed in other known phases of 2D silica. This structure consisted of bond distortions in silica (from hybridization), while maintaining the overall lattice.
The authors conclude, “With 2D materials serving as a creative sandbox for studying emergent properties, demonstrating such one-pot, large-area growth processes to construct 2D hybrids from the bottom up opens new doors to true materials discovery with scalability.”
While this study was led by Rice University researchers, in the press release, Iyengar emphasizes that this study “was not something only one lab could do.”
“This research was a cross-continental effort” involving colleagues from The Pennsylvania State University (U.S.), University of Sussex (U.K.), and Federal University of Minas Gerais (Brazil), Iyengar says, and it “opens the door to combining entirely new classes of 2D materials.”
The open-access paper, published in Advanced Materials, is “Glaphene: A hybridization of 2D silica glass and graphene” (DOI: 10.1002/adma.202419136).
Author
Laurel Sheppard
CTT Categories
- Manufacturing
- Material Innovations
- Nanomaterials
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026