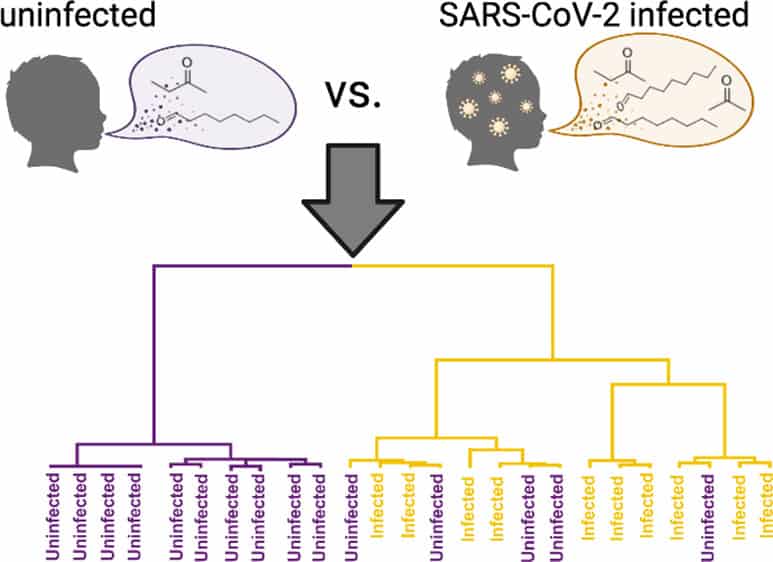
[Image above] Researchers in Pennsylvania identified six candidate biomarkers that indicate SARS-CoV-2 infection in children. Credit: Berna et al., ACS Infectious Diseases (CC BY-NC-ND 4.0)
As colleges and universities prepare for the start of fall semester, the logistics of surveillance testing for COVID-19 are again at the forefront of many administrators’ minds, especially as states pass laws banning schools from requiring vaccinations and masks.
Administrators must consider a variety of factors when designing a testing strategy, including deciding how much time and how many resources to devote to the tests. They also must decide how the tests will be administered, through either a nasal swab, throat swab, saliva sample—or, possibly in the near future, a breathalyzer.
Breathalyzers are devices that detect chemical compounds in the moisture of someone’s breath. The first breathalyzers developed in the early- to mid-1900s were designed to detect the amount of alcohol in someone’s breath (and thereby estimate their blood alcohol concentration). Now, researchers are designing breathalyzers to monitor a variety of other health conditions, including sleep disorders, liver disease, and diagnosing people with COVID-19.
We wrote an in-depth CTT last June on work by Perena Gouma, Edward Orton, Jr., Chair in Ceramic Engineering and director of the Advanced Ceramics Research Laboratory at The Ohio State University, on developing a COVID-19 breathalyzer. A recent article by The New York Times this July highlights other research groups around the world investigating COVID-19 breathalyzers as well.
All these breathalyzers rely on the fact that SARS-CoV-2 infection alters the amount of certain chemical compounds in someone’s breath. By testing for the levels of these compounds, the breathalyzers can diagnosis someone with COVID-19 quite reliably.
However, while the breath profile is fairly well-mapped for adults, few studies have investigated how a SARS-CoV-2 infection may affect the breath profile of children.
“Children with SARS-CoV-2 infection typically experience mild symptoms of disease and are much less likely to experience severe outcomes, such as hospitalization or death, as compared to adults. Children also exhibit a distinct immunological response to coronavirus infection. These clinical and immunological differences in children with SARS-CoV-2 suggest that their metabolic response to infection may also be different than that of adults,” researchers write in a recent open-access paper.
The researchers come from Children’s Hospital of Philadelphia and University of Pennsylvania. In their study, they conducted 2D gas chromatography and time-of-flight mass spectrometric analysis of 84 breath volatiles in breath samples from two independent cohorts of children with and without SARS-CoV-2. They identified six candidate biomarkers that indicate SARS-CoV-2 infection in children—most of which were different from the candidate biomarkers established for adults.
“Two of these markers (octanal and heptanal) were also elevated in the breath of adults with the disease, while the others were unique to infected children,” an American Chemical Society press release explains.
The researchers then analyzed breath samples from a different group of 24 children to establish reproducibility of the six candidate biomarkers, which proved successful—the biomarkers predicted infection with 91% sensitivity and 75% specificity.
“Thus, our breath biomarkers approach the WHO priority product profile for COVID-19 diagnostics (‘acceptable’ sensitivity ≥80% and specificity ≥97%) for point-of-care testing, for suspected COVID-19 cases where RT-PCR cannot be delivered in a timely manner,” they write.
The open-access paper, published in ACS Infectious Diseases, is “Reproducible breath metabolite changes in children with SARS-CoV‑2 infection” (DOI: 10.1021/acsinfecdis.1c00248).
Author
Lisa McDonald
CTT Categories
- Material Innovations