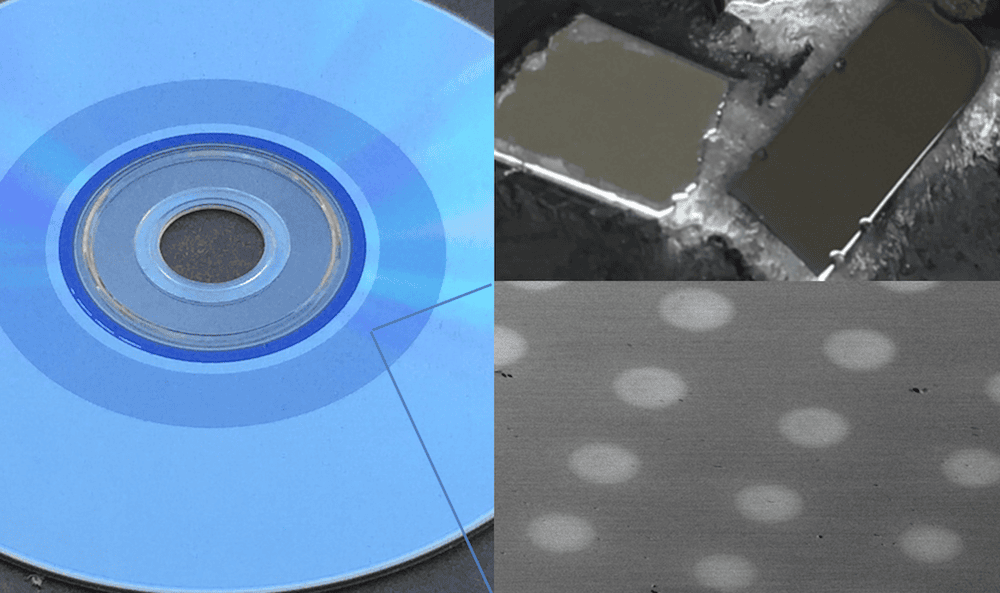
[Image above] What is inside a DVD? Memory phase change materials, which quickly switch between glassy and crystal phases (upper right) to store data on the disc. Bottom right shows crystallized dots on an amorphous film as in a DVD. Credit: Pierre Lucas
What is the best way to store data?
If you are looking for archival storage, magnetic tape is a pretty good choice. But if you are looking for a device that offers fast computing, then devices based on memory phase change materials (PCMs) may be your material of choice.
Memory PCMs are materials that store data by quickly switching between a glassy phase and a crystal phase. In an email, ACerS member Pierre Lucas, professor of materials science and engineering at the University of Arizona, explains the process.
“[PCMs] unique feature is their ability to switch from glass to crystal to encode the 0 and 1 (digital information) in your computer and DVD,” he says. “Optical memories work by switching between the low reflectivity glass bit (0) and the high reflectivity crystal bit (1), while computer memories work by switching between the low conductivity glass bit (0) and the high conductivity crystal bit (1).”
Compared to conventional dynamic random access memory (DRAM) based on capacitors, which require continuous electrical current to retain memory, PCM memory is often called “nonvolatile memory” because it remains permanent when the power is turned off. Additionally, PCMs are extremely fast and can switch in a few nanoseconds for fast computing.
Using PCMs for data storage is not a new concept. We wrote about such a use in 2011, and even then memory PCMs had been in use commercially for years in optical read-write applications (like DVDs and Blue Ray discs) and showed promise in electronic memory systems (like cell phones).
All commercial memory PCMs are made of the chalcogenides germanium antimony telluride (Ge-Sb-Te) or doped antimony telluride (Sb-Te).

Since 1997, DVDs have held a huge role in home entertainment. Better understanding of memory PCMs can improve the technology even further. Credit: PxHere
Chalcogenides are ideal candidates for data storage because they have
- high contrast in conductivity and reflectivity between crystalline and glassy phases,
- rapid and reversible transformation between these two phases at elevated temperatures, and
- stability of the glassy phase near room temperature (which ensures data retention).
However, there is an obstacle to using the chalcogenide Ge2Sb2Te5 for data storage.
To control the switch between glassy and crystal phases, you need to know the glass transition temperature (Tg), or the temperature below which the atoms of an undercooled liquid transition to a glassy state (temporarily freeze without crystallizing). Yet Ge2Sb2Te5 does not exhibit a well-defined Tg.
The lack of a defined Tg for Ge2Sb2Te5 has led to contradicting interpretations regarding the state—glassy or undercooled liquid (UCL)—from which the material crystallizes.
“Unambiguous experimental evidence for either possibility is currently missing since kinetic measurements alone do not permit clear characterization of a glass transition,” researchers write in a recent open-access paper.
The researchers, including Lucas and others from professor Matthias Wuttig’s group at RWTH Aachen University in Germany, looked to characterize thermodynamic and kinetic signatures of the transition to unambiguously reveal the nature of the phase transition.
To characterize both thermal and kinetic signatures, the researchers investigated a broad range of heating rates spanning six orders of magnitude using a combination of conventional and ultrafast differential scanning calorimetry (DSC and FDSC). They combined this information with numerical simulations of crystallization to determine from which state—glassy or UCL—crystallization occurs.
They found Ge2Sb2Te5 crystallizes from the glassy phase below a heating rate of 10,000 K/s. Between 104 and 105 K/s, they saw a transition from glass-like to UCL-like temperature dependence of crystallization. Over 100,000 K/s, they determined crystallization occurs completely from the UCL state.
Lucas says their finding is surprising. “Normally you need to raise the temperature above the glass transition in the liquid state to observe crystallization,” Lucas says. “In the case of phase change materials Ge2Sb2Te5, we found that the glass is so unstable that it can undergo fast crystallization … [when] it is still technically a solid. This is highly unusual and unexpected from a glass.”
The open-access paper, published in Advanced Materials, is “Switching between crystallization from the glassy and the undercooled liquid phase in phase change material Ge2Sb2Te5” (DOI: 10.1002/adma.201900784).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Glass


