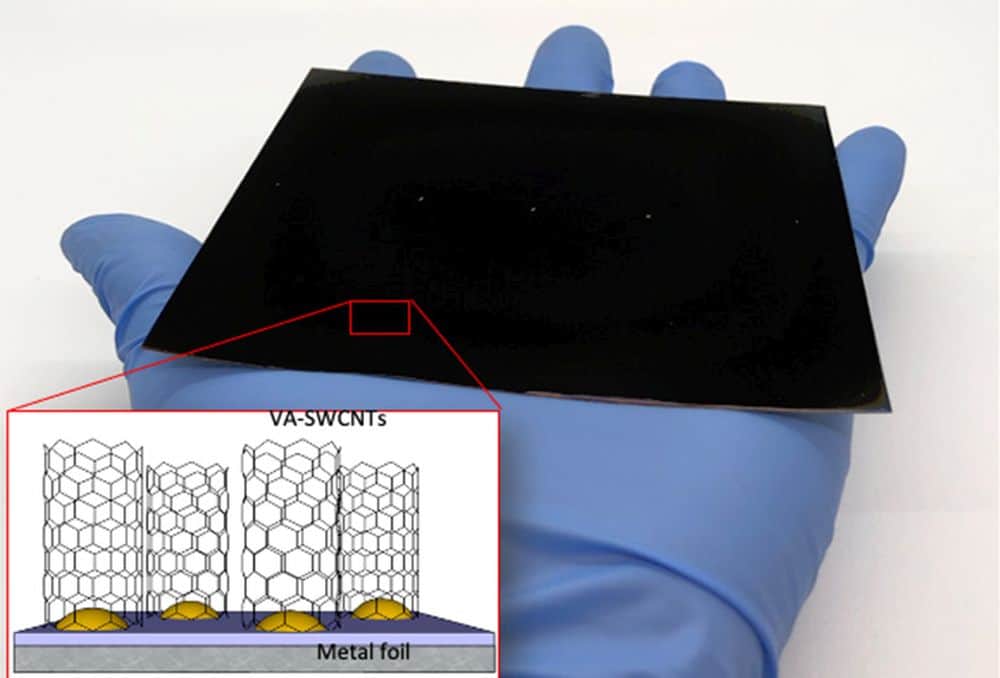
[Image above] Photograph and schematic representation (inset) of a scaled-up sample of vertically aligned single-walled carbon nanotubes grown on Inconel metal foil. Credit: Lawrence Livermore National Laboratory
Carbon nanotubes (CNTs) are often described as rolled up sheets of graphene, but this imagery does not accurately convey how CNTs are fabricated.
Typically, CNTs are grown vertically on a substrate through chemical vapor deposition (CVD). This process involves flowing gaseous carbon-containing molecules over a substrate containing catalytic particles. The catalysts decompose the carbon-containing gas, freeing carbon atoms to build up in vertical tubes.
CVD provides accurate control over the physical characteristics of CNTs, such as nanotube length, diameter, and lattice orientation. However, achieving mass production with this fabrication method is currently not possible due to several factors, including choice of substrate.
Traditional insulating substrates used in CVD, such as silicon and quartz, are not compatible with automated batch systems or roll-to-roll lines for low-cost mass production. In contrast, metal foils can be easily integrated into large-scale (semi)continuous manufacturing processes. But foils have less ideal chemical, physical, and morphological features, for example, a rough and conductive surface.
Numerous studies have explored ways to overcome these challenges with flexible metal substrates. Now, three researchers at Lawrence Livermore National Laboratory published a review paper summarizing these efforts. Highlights from the 20-page paper are below.
Metal substrate selection
An early work by Hiraoka et al. (2006) revealed that many readily available pure metal foils present hurdles to growing single- and double-walled CNT via CVD. For example, aluminum foil melted at the standard growth temperature; silver and copper foils did not yield CNT growth; and molybdenum, tantalum, and niobium became brittle upon exposure to the CVD growth conditions.
Despite these challenges, many of these and other metal foils remain heavily studied today. For example, Inconel (a nickel-chromium-based superalloy), copper, nickel, stainless steel, aluminum, iron–chromium–aluminum alloy, and constantan (a copper–nickel alloy) are among the most studied ones.
For some metals, such as stainless steel, the foil can be used as-is for the substrate. But in many cases, a barrier layer must be deposited on the foil to prevent the metal from diffusing and detrimentally changing the catalyst layer chemistry.
Together with composition, surface roughness is a significant parameter that affects a foil’s ability to support CNT growth. This statement is true even when the foil is used simply as a support for a catalyst stack rather than as the catalyst itself.
Materials for barrier layers and catalysts
Researchers have used many different materials as the barrier layer to sustain CNT growth on metal foils, including alumina, aluminum, silicon oxides, titanium, titanium nitride, nickel, Inconel, and manganese oxide.
Alumina is the most common barrier layer used in both traditional CNT growth on silicon wafers and on metal substrates. It can effectively support different types of metal catalysts, mitigate subsurface diffusion, and thus control CNT diameters.
A thicker barrier layer is usually needed to overcome the surface roughness. However, in another recent study by the authors, they successfully grew uniform CNT forests on Inconel foils with an alumina barrier layer of 10 nm, and the CNTs maintained the same structural properties as those grown on foils with a thicker barrier layer (40 nm).
As noted earlier, sometimes the metal foil itself can be used to initiate catalytic activity without additional barrier or catalyst layers. However, pretreatments are often required to ensure efficient growth on catalyst-stack-free metal substrates. As such, catalysts are typically stacked on top of the metal foils, with iron deposited on an alumina barrier layer being the most popular choice.
Methods for depositing barrier layers and catalysts
Methods for depositing barrier layers and catalysts on metal foils can generally be classified as physical vapor deposition (PVD), atomic layer deposition (ALD), or electrochemical processes.
PVD techniques are the traditional deposition approach. Out of the ones described in this paper, sputtering is the most amenable to integration into roll-to-roll manufacturing due to its ability to operate at higher pressures. However, PVD techniques suffer from limited scalability due to a reduced deposition rate, nonuniform evaporation rate, and difficulty with coating inner surfaces of complex geometries.
Unlike the PVD techniques, ALD allows for conformal deposition even on high-aspect-ratio features and tunable layer thickness for planar and multifaceted surfaces. But though ALD can operate at atmospheric pressure, it is not an easily scaled process.
Electrochemical techniques are low-cost, efficient, and scalable. But these methods have only been used successfully to deposit catalysts, not barrier layers, and the resulting CNTs are typically multiwalled rather than single walled.
Other solution-based deposition techniques on metal substrates include drop, spray, spin, and dip-coating processes. Though these techniques are not as reproducible or uniform as ultrasonic spray pyrolysis, another solution-based process, they are affordable and scalable.
Future directions
The authors note that some companies have recently demonstrated bulk production of CNTs for various applications, such as coatings and adhesives, plus commercial roll-to-roll tools are being developed to integrate CVD growth methods into manufacturing lines.
“This continuous progress toward advanced manufacturing of CNT arrays on flexible metal substrates is expected not only to enable large-scale production of CNTs at a fraction of the cost, but also revolutionize countless technologies ranging from energy storage and electronics to lightweight multifunctional composites, smart textiles, and membranes,” they conclude.
The paper, published in Carbon, is “Growth of carbon nanotube forests on flexible metal substrates: Advances, challenges, and applications” (DOI: 10.1016/j.carbon.2023.02.054).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Education
- Nanomaterials


