
[Image above] Travis Olds, left, and Lisa Haney with powders that they and colleagues at Washington State University created based on research into the world’s first synthetic pigment, Egyptian blue. Credit: Joshua Franzos, Carnegie Museum of Natural History
Purple is my favorite color, and I even have a shade of it highlighting my hair (at my age you don’t care what other people think!). But globally, blue is considered the most popular color, especially in the countries of Australia, China, Germany, and the United States.
Why is blue so popular? It may be because this color is associated with trust and security. Whatever the reason, it is interesting to note that there was no word for blue in most ancient cultures’ vocabularies. Blue was rarely found in nature (aside from the water and the sky); instead, brown, red, yellow, and green colors dominated.
The prevalence of blue in human culture changed about 2500 BCE when the Egyptians became the first culture to develop a synthetic pigment: a blue dye of various tones, similar in color to the stones lapis lazuli (first mined in what is now Afghanistan circa 7000 BCE) and turquoise (first mined in the Sinai Peninsula of Egypt circa 5000 BCE). These stones were expensive and could only be made into objects or inlays, so an alternative way to add pretty blue colors to objects was needed.
The Egyptians discovered that by heating a mixture of lime, sand, a copper compound, and an alkaline flux, they could create a calcium copper silicate material with a stable blue color. By grinding and mixing this pigment with a simple binder, it then could be applied to wood, stone, and cartonnage (a layered material made with linen, such as papier-mâché). It could also be used to decorate beads and amulets.
Because Egyptian blue cost less than the decorative stones and was easier to apply, it became popular around the Mediterranean region for more than 3,000 years. Its popularity eventually waned, however, and the pigment went out of use during the Renaissance (14th–17th centuries).
In recent years, there has been a resurgence of interest in Egyptian blue due to its interesting optical, magnetic, and biological properties. For example, it absorbs light broadly in both the visible and near infrared spectrum (between 430–800 nm). It also emits a luminescence band at about 910 nm, which allows modern art conservators and scientists to identify Egyptian blue even when the color is obscured or degraded.
This renewed interest in Egyptian blue inspired a team of researchers led by Washington State University, in collaboration with Carnegie Museum of Natural History and the Smithsonian’s Museum Conservation Institute, to recreate Egyptian blue. The goal was to better understand the procedures that early cultures used to manufacture Egyptian blue by examining the effect of precursor selection on process variability.
As noted before, Egyptian blue is a calcium copper silicate material, with the main coloring agent being cuprorivaite (CaCuSi4O10). The pigment can be quite heterogeneous, however, and commonly includes other materials such as silicate glass, the minerals cristobalite and tridymite, and sometimes wollastonite (CaSiO3). These inclusions affect the final tone of the pigment, allowing it to range from deep blue to dull gray or green.
To better understand the effect of these inclusions on the final blue tone, the researchers created 12 different recipes of Egyptian blue using crystalline silicon dioxide, a calcium source, and various copper sources, including copper oxide and natural secondary copper carbonate minerals (azurite or malachite). Several recipes included additional sodium carbonate.
Heating of the mixtures took place at about 1,000°C for between one and 11 hours in a high-temperature box or muffle furnace. This temperature matches the range that other researchers have suggested the Egyptians used. A slightly higher temperature and longer reaction time also promoted the solid-state formation of cuprorivaite.
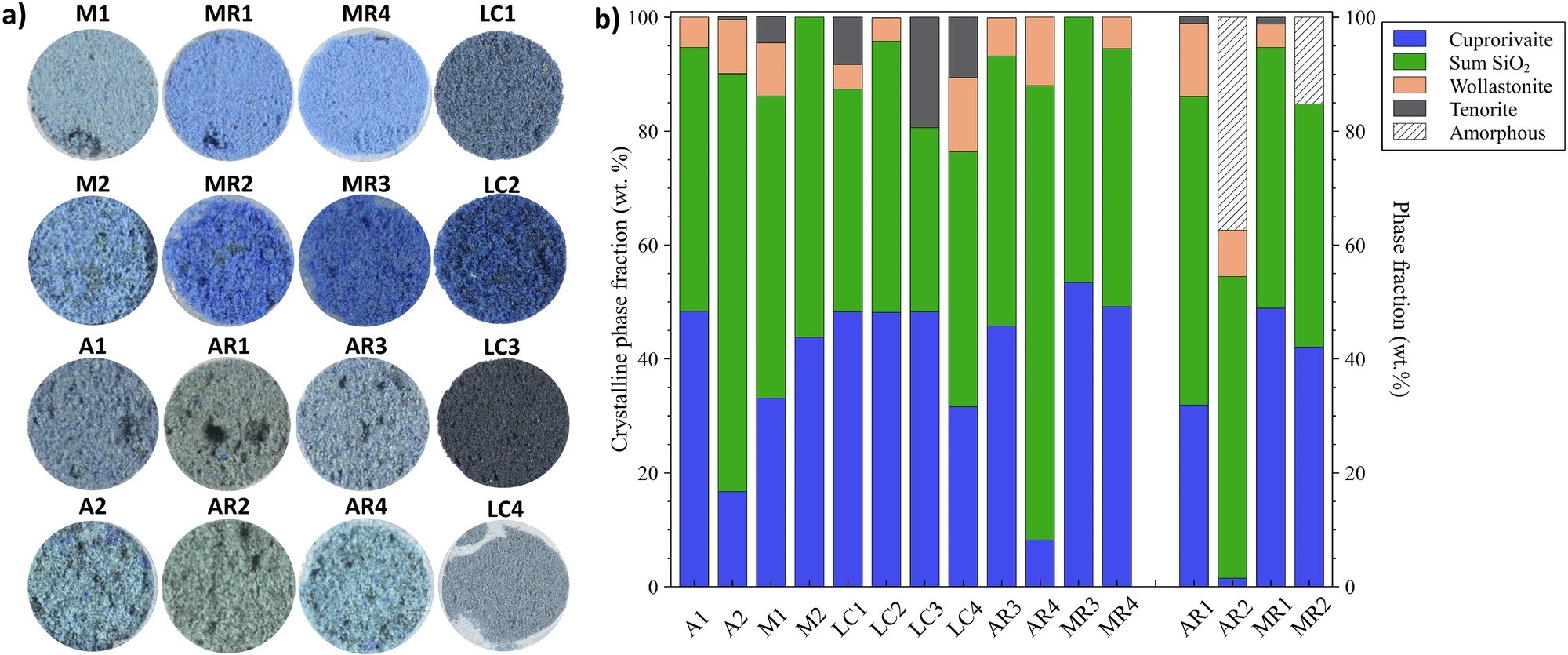
a) Optical image and b) quantitative X-ray diffraction of the synthesized Egyptian blue samples. Values to the left of the diffraction pattern are standardless (only crystalline phases are quantified); values shown at right for AR1, AR2, MR1, and MR2 include an internal zinc oxide standard so the amorphous phase is additionally properly quantified. Credit: McCloy et al., npj Heritage Science (CC BY-NC-ND 4.0)
After cooling the samples at various rates, the researchers investigated the chemical composition, phases, and microstructure of the particles using various characterization and imaging techniques.
Powder X-ray diffraction established the presence of cuprorivaite as the dominant crystalline component, as well as the presence of unreacted or thermally altered silica polymorphs, including quartz, cristobalite, and tridymite. Most of the samples also contained wollastonite. Laser Raman spectroscopy and photoluminescence measurements confirmed these phases identified by X-ray diffraction.
Visible to near-infrared reflectance measurements were made on all synthesized pigments. These data showed strong reflectance in both the visible (blue) and near-infrared regions.
Comparison with ancient Egyptian blue pigments found in artifacts at Carnegie Museum of Natural History and in the literature also confirmed that a multiphase mixture (cuprorivaite intermixed with one or more silica polymorphs, wollastonite, tenorite, and/or silicate glass) was the “rule rather than the exception,” the researchers write in the open-access paper describing their experiments.
Other conclusions based on these analyses:
- The effect of particle size on measured and perceived color was significant, with larger particles producing a deeper blue.
- The copper precursor had a large effect on the overall color obtained. Malachite produced a bright blue color after only one hour of heat treatment; under the same conditions, azurite produced a gray–green color.
- To obtain the bluest color only required about 50% of the blue-colored components. If copper-bearing wollastonite and glass were present, the average color shifted more toward green–yellow and away from blue–red.
- Longer treatment at high temperature and slower cooling produced bluer pigments because the cuprorivaite concentration increased at the expense of the glass phase and silica.
The researchers conclude that further work is required to quantify the effect of wrapping, parchment, and paint binder on the substrate’s coloration for the range of Egyptian blues encountered in heritage objects.
Regardless, “We hope this [paper] will be a good case study in what science can bring to the study of our human past,” says ACerS Fellow John McCloy, first author and director of Washington State University’s School of Mechanical and Materials Engineering, in a news release.
The newly synthesized Egyptian blue samples are currently on display at Carnegie Museum of Natural History as part of the Stories We Keep exhibition. In late 2026, these samples will be placed into a permanent display as part of the museum’s new long-term gallery focused on ancient Egypt.
The open-access paper, published in npj Heritage Science, is “Assessment of process variability and color in synthesized and ancient Egyptian blue pigments” (DOI 10.1038/s40494-025-01699-7).
Author
Laurel Sheppard
CTT Categories
- Art & Archaeology


