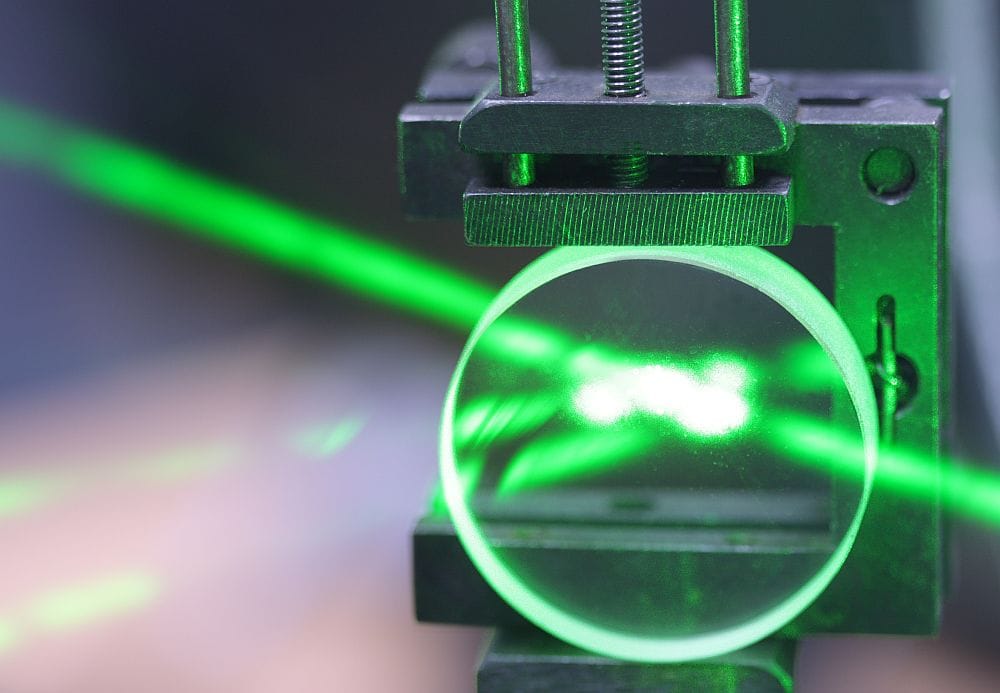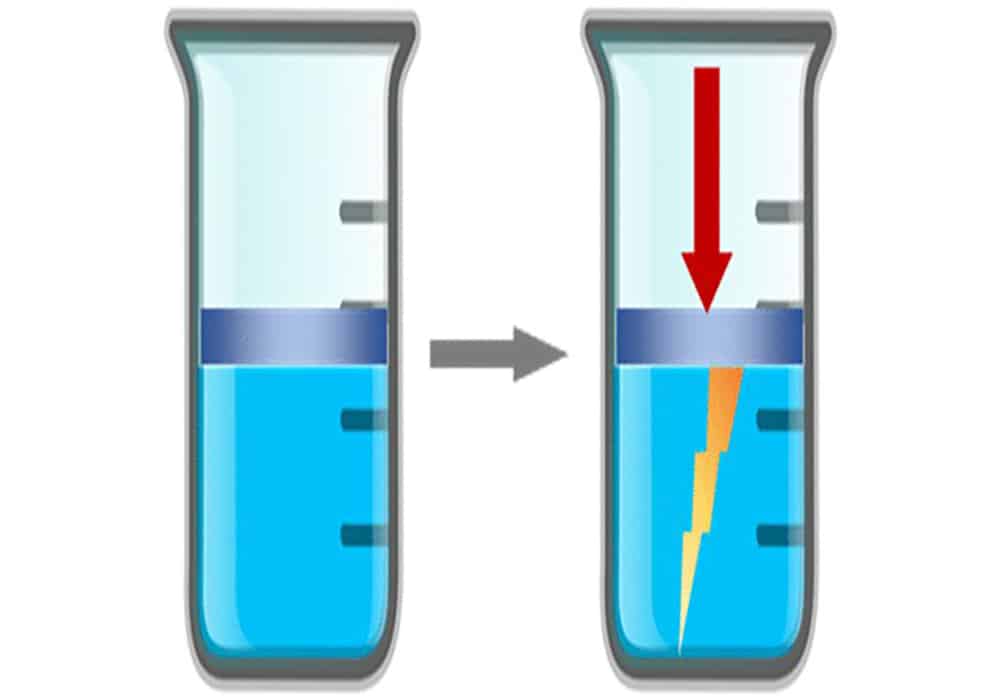
[Image above] Illustration of an ionic liquid being compressed by a piston in a cylinder, resulting in the generation of an electric charge. Reprinted with permission from Hossain and Blanchard, The Journal of Physical Chemistry Letters. Copyright 2023 American Chemical Society.
As you bust your old gas grill out of storage to prepare for the upcoming BBQ season, be sure to thank ceramics—specifically the piezoelectric igniter—for making this tradition possible.
Piezoelectricity refers to the generation of an electric charge in certain materials resulting from applied mechanical stress. Materials that exhibit this “direct” piezoelectricity also exhibit “converse” piezoelectricity, i.e., the generation of mechanical strain by applying an electric field.
The piezo igniters found in grills are just one example of the many ways piezoelectricity is harnessed in today’s world. Other applications include industrial actuators, medical sensors, and even instrument pickups.
While ceramics account for most commercially relevant piezoelectric materials, piezoelectric behavior is also observed in many polymers, as well as biological molecules such as DNA, viral proteins, and amino acids. Yet all these diverse materials share one thing in common—they manifest piezoelectricity while in the solid phase.
Current understanding of the mechanisms behind piezoelectricity requires a material to have substantial structural organization. It is not surprising, then, that all known piezoelectric materials are solids because liquids and gases are generally considered to have no persistent order.
“As a consequence, one would hardly ever think to look for a piezoelectric response from a liquid,” says Gary Blanchard, professor of chemistry at Michigan State University.
Yet this basic assumption was turned on its head in a new paper published by Blanchard and Ph.D. candidate Md Iqbal Hossain. Their paper reports the observation of piezoelectricity in liquids for the first time, specifically room-temperature ionic liquids.
Ionic liquid is the term for a salt in the liquid state. Unlike conventional liquids such as water and gasoline, which are predominantly made of electrically neutral molecules, ionic liquids consist of ions and have a net electrical charge.
Ionic liquids are recognized as highly promising alternative green solvents in chemical processes. They also have been used as additives in advanced (solid-phase) materials, such as perovskite solar cells.
In an IEEE Spectrum article, Blanchard says his group was conducting experiments designed to better understand the basic properties of ionic liquids. They were shocked to find two different room-temperature ionic liquids each generated electricity when a piston squeezed them within a cylinder.
Blanchard and his students repeated the experiment multiple times to confirm reproducibility of the results. They also filled the cylinder with two standard liquids, ethylene glycol and 1 M NaCl in ethylene glycol, to confirm neither produced a measurable piezoelectric response when compressed.
With this validation, they then tested if the room-temperature ionic liquids exhibited converse piezoelectricity. They did so by applying an electric charge to ionic liquids stored in a lens-shaped container. Upon application, there was a measurable change in the ionic liquid’s focal length—i.e., how much it bent incoming light—which suggests the liquid experienced mechanical strain.
Blanchard says they are still trying to figure out the fundamental mechanisms behind the piezoelectric effect in these liquids. What is certain, though, is that “the current theory for solid-state piezoelectric materials will require some modification to account for the experimental observations presented here,” he and Hossain write in the paper.
The magnitude of the piezoelectric effect in these ionic liquids is an order of magnitude smaller than that of quartz, a widely used piezoelectric ceramic. Blanchard says it will be interesting to see if other ionic liquids have a bigger effect.
The paper, published in The Journal of Physical Chemistry Letters, is “Ionic liquids exhibit the piezoelectric effect” (DOI: 10.1021/acs.jpclett.3c00329).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Material Innovations