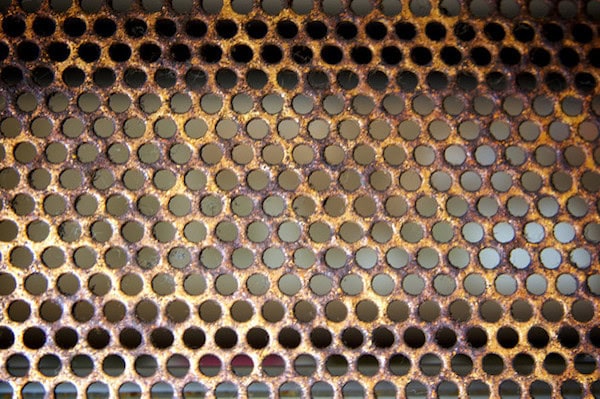
[Image above] Credit: Jeremy Brooks; Flickr CC BY-NC 2.0
There is no shortage of materials that are capable of being recycled or repurposed. We can recycle plastic bottles, aluminum, paper, cardboard, glass, steel—even batteries and electronics.
And we all win when recycling companies work their magic and turn our discarded items into useful products.
It’s also a good thing that scientists are developing new methods to recycle common everyday things, such as glass bottles, plastic bottles, and especially batteries in our electronics.
Researchers in China have found a use for a material that you would not typically think of as something to be recycled—rusty stainless steel mesh. They are turning the material into battery electrodes for use in potassium-ion batteries. (And, for those who don’t believe stainless steel can rust, here are several scenarios that say otherwise).
XinBo Zhang, professor at the Chinese Academy of Sciences, and his research group have previously researched forms of renewable energy in batteries, including lithium-ion, sodium-ion, and metal-air batteries.
Lithium-ion batteries have the highest energy density of other battery types and are used to power things as small as mobile devices and as large as jet aircraft. Although they dominate the industry, they do have their challenges, as we are well aware—plus, lithium is becoming more expensive.
Sodium-ion batteries are a less expensive and safer alternative to lithium-ions, and Zhang’s group has also explored carbon nanosheets as a viable electrode material for sodium-ion batteries.
Now, the group is experimenting with using potassium ions in batteries. “Potassium ions are just as inexpensive and readily available as sodium, and potassium-ion batteries would be superior from the electric aspect,” Zhang explains in a press release.
But he says the problem with potassium ions is that they tend to destabilize electrode materials with the recurring storage/release cycle of ions.
An environmentally friendly solution
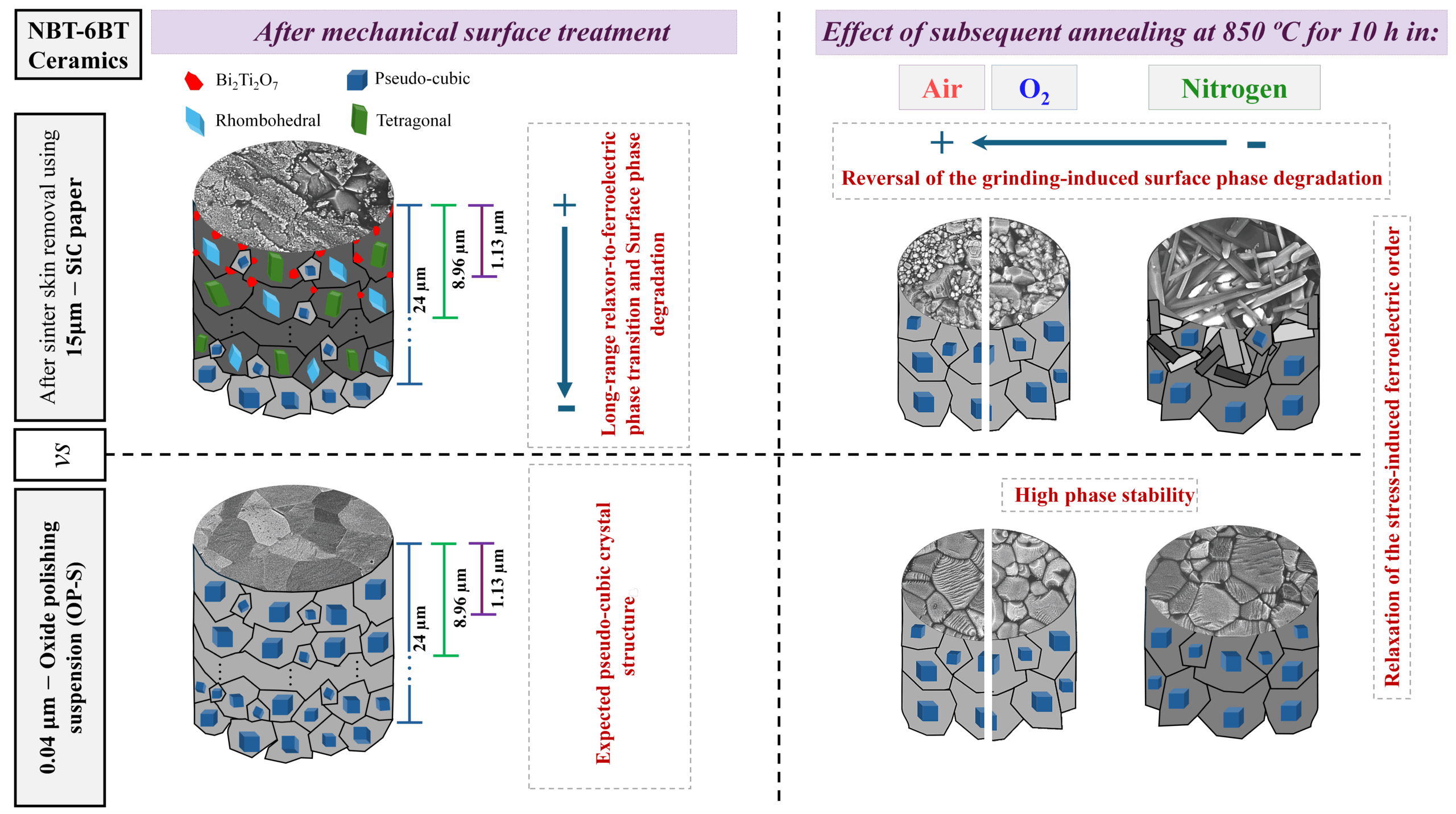
His team seems to have solved this by making electrodes out of discarded corroded stainless steel mesh filters and sieves. Instead of the costly and polluting process of recovering the metal using a furnace, Zhang’s group used a chemical solution of potassium ferrocyanide to dissolve the rust. During the chemical reaction, the metal ions combine with ferricyanide ions to form a dark blue pigment, called Prussian blue.
The result is cubic scaffold nanocubes where potassium ions can be stored, waiting to be released. They then add a layer of graphene oxide on to the nanocubes, which converts to reduced graphene oxide and “inhibits clumping and detachment of the active material,” Zhang says. “At the same time, it significantly increases the conductivity and opens ultrafast electron-transport pathways.”

Credit: Wiley newsroom
So not only are the researchers reusing a material that might have ended up in a landfill, they’re making a lower-cost, high-performance battery that could someday replace more expensive battery materials. That’s a solution we can all look forward to.
The paper, published in Angewandte Chemie is “Transformation of rusty stainless-steel meshes into stable, low-cost, and binder-free cathodes for high-performance potassium-ion batteries” (DOI: 10.1002/anie.201702711).
Author
Faye Oney
CTT Categories
- Basic Science
- Electronics
- Energy
- Environment
- Material Innovations
- Nanomaterials