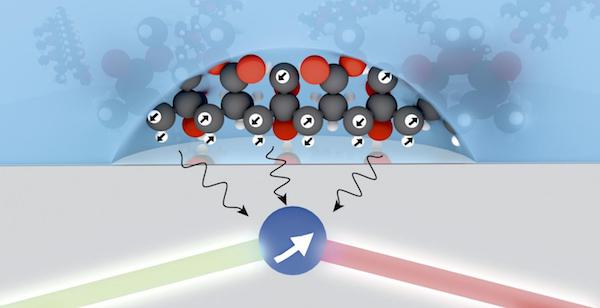
[Image above] Credit: David A. Broadway; cqc2t.org
Diamonds may be a girl’s best friend, but imperfect diamonds might be a scientist’s best friend.
Australian researchers at the Centre for Quantum Computation & Communication Technology (CQC2T) have improved the ability to characterize molecules with spectroscopy—and the key to their technique is a diamond with a flaw.
Led by Lloyd Hollenberg, professor of physics at the University of Melbourne, the research team’s goal was to be able to view objects more accurately at the nanoscale level without using microwaves that are typically required for nuclear magnetic resonance (NMR) spectroscopy.
“This quantum probe delivers a dramatic improvement in NMR technology,” Hollenberg explains in a news release. “In addition to being able to detect NMR in far smaller samples than conventional machines, our technique does not require the application of microwave fields that might disrupt biological samples.”
This is important because current NMR methods use microwave rays that can damage molecules during analysis. Also, larger machines require significantly more spins of the nuclei of atoms in order to detect a magnetic signal. Which of course, uses more power.
Additional problems with larger NMR machines have to do with distance between the machine and what it’s measuring, as well as small size of the signals, Alastair Stacey, one of the CQC2T researchers says. “The problem with the large NMR machines in widespread use today is that the signals we’re trying to detect are extremely small, and the distance from the measurement device to the object being measured is very large.”
So results are less accurate, because the machine cannot obtain a detailed picture of molecular activity. It’s like the difference between watching a football game from the nosebleed section and watching it through binoculars.
Peering through a diamond’s flaw
According to an article from News Corp Australia Network, a diamond with a flaw is missing a carbon atom, which creates a tiny hole. A nitrogen vacancy occurs when a nitrogen atom sits adjacent to the hole. “The atom-sized hole can capture two electrons which—with additional light—act as a high resolution ‘probe’ to the quantum world.”
This enables the researchers to measure signals from smaller numbers of nuclear spins, allowing them to see higher quality images in smaller sample sizes.
Like looking through binoculars.
Hollenberg says that using the quantum properties of a diamond’s defect can help them identify “much smaller volumes down to only thousands of spins.”
Their discovery could prove valuable for drug research in understanding interactions between molecules and the biosamples being tested.
The paper, published in Nature Communications, is “Microwave-free nuclear magnetic resonance at molecular scales” (DOI: 10.1038/ncomms15950).
Author
Faye Oney
CTT Categories
- Basic Science
- Nanomaterials
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026


