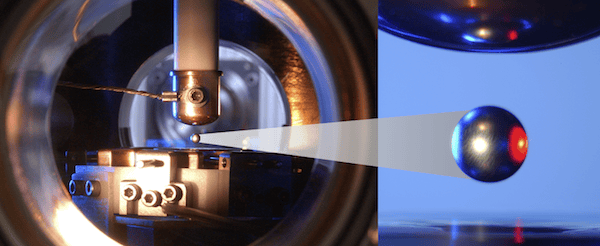
[Image above] Molten oxide droplet levitated in the electrostatic levitation furnace at Marshall Space Center. Credit: Marshall Space Flight Center
I’ve got it in my head that I need to replace my smartphone. There’s nothing wrong with my ancient (eyes rolling), 2-year-old iPhone 5. It’s actually in great shape and works just fine.
No—I need the Galaxy Note7, just released by Samsung, because it will allow me to be an “early adopter” and brand loyal.
But not in the way you might expect.
The Galaxy Note7 is the first device to incorporate Corning’s new Gorilla Glass 5. My consumer loyalty attaches to the glass technology and brand, not the device technology and brand.
Call me a glass nerd—I’ll wear the label with pride.
Samsung’s marketing doesn’t mention Gorilla Glass 5 directly. It’s an interesting indicator of consumer expectations for miraculous glass performance without a clue about the science and engineering behind it. But you and I know there’s plenty of both.
What will it take to develop the next generation of display glasses or other types of high-performance glasses? For example, the pharmaceutical industry needs glasses that resist degradation from increasingly caustic chemotherapy drugs. (Think about that for a sobering moment.)
As with most materials, structure dictates properties, but in the case of glasses, characterizing structure is elusive because it’s amorphous and impacted by almost everything: composition, thermal history, dimension, time, etc. Motivated by the need to understand and manipulate glass structure, glass researchers have sought accurate modeling strategies to drive innovation in the glass industry and its search for new compositions and better processes.
Topological constraint theory (pdf) has emerged as the predominant modeling tool. The underlying premise treats the glass network as nodes constrained by the rigid rods (bonds) that join them. An important factor to consider, though, is the temperature-dependence of the localized “rigid rod” structure.
In a recent paper, Materials Development Inc. (Arlington Heights, Ill.) approached the problem from a different theoretical angle. Oliver Alderman, lead author and R&D scientist at MDI, says in an email, “The powerful theory of topological constraints is unable to predict a broad maximum in sodium borate glass transition temperatures in the 20–50 mol% Na2O composition region.”
Alderman et al. wanted to see whether approaching structure from the theoretical perspective of the thermodynamic model of ideal associated solutions could describe temperature-dependent structure changes. According to the paper, ideal associated solutions “describe an oxide liquid as an ideal solution of end member oxides and any stoichiometric compounds forming within the system.”
These investigations have commercially significant implications. Alderman says, “Structure dependent properties such as melt viscosity play a crucial role in glass making.” In addition, sodium borates are important end members of sodium borosilicate glasses with widespread commercial applications, such as Pyrex labware and optical components.
The team used X-ray diffraction on stoichiometric Na2B4O7 melts suspended in an aerodynamic levitation furnace and, indeed, showed “the existence of a continuous structural transition… and that this can be reasonably well predicted by the thermodynamic model of ideal associated solutions.”

Droplet suspended between electrodes in an electrostatic levitation furnace. Credit: Marshall Space Flight Center
“However,” Alderman notes in the email, “there are several cases where the thermodynamic model can be seen to fail, presumably due to non-ideal mixing of the stoichiometric groupings. In the future it would be nice to see if there is any way to account for this and broaden the reach of the model and ideally try to combine it with constraint theory to get the best of both.”
A new MDI initiative may generate the data needed to unlock some of those mysteries. The company just learned that it won one of 16 research slots on the International Space Station. Using the electrostatic levitation furnace onboard, MDI will be able to precisely measure thermophysical properties of molten metal oxides as they supercool. “The value of low gravity for this work is the lack of unwanted fluid motion that will allow us to make very precise measurements in diffusion controlled conditions,” says Richard Weber, MDI founder and CEO, in an email.

Levitated molten drop viewed through the port of an electrostatic levitation furnace. Credit: Marshall Space Flight Center
The NASA-funded project is expected to begin fall 2016 and run four years. Weber says MDI will initially work on ground-based experiments using MDI and NASA levitation instruments for about two years to fine-tune compositions and experimental protocols, anticipating flight time in the third year.
Meanwhile, Weber says, “We will continue our separately funded ground-based work on melt structure at the Advanced Photon Source at Argonne National Lab. One mid-term goal is to start linking melt structure, properties, and glass forming behavior to get a better understanding of the glass formation process.”
The paper, published in the Journal of Physical Chemistry C, is “Temperature-driven structural transitions in molten sodium borates Na2O–B2O3: X-ray diffraction, thermodynamic modeling, and implications for topological constraint theory” (DOI 10.1021/acs.jpcc.5b10277) (subscription required).
Author
Eileen De Guire
CTT Categories
- Glass
- Modeling & Simulation


