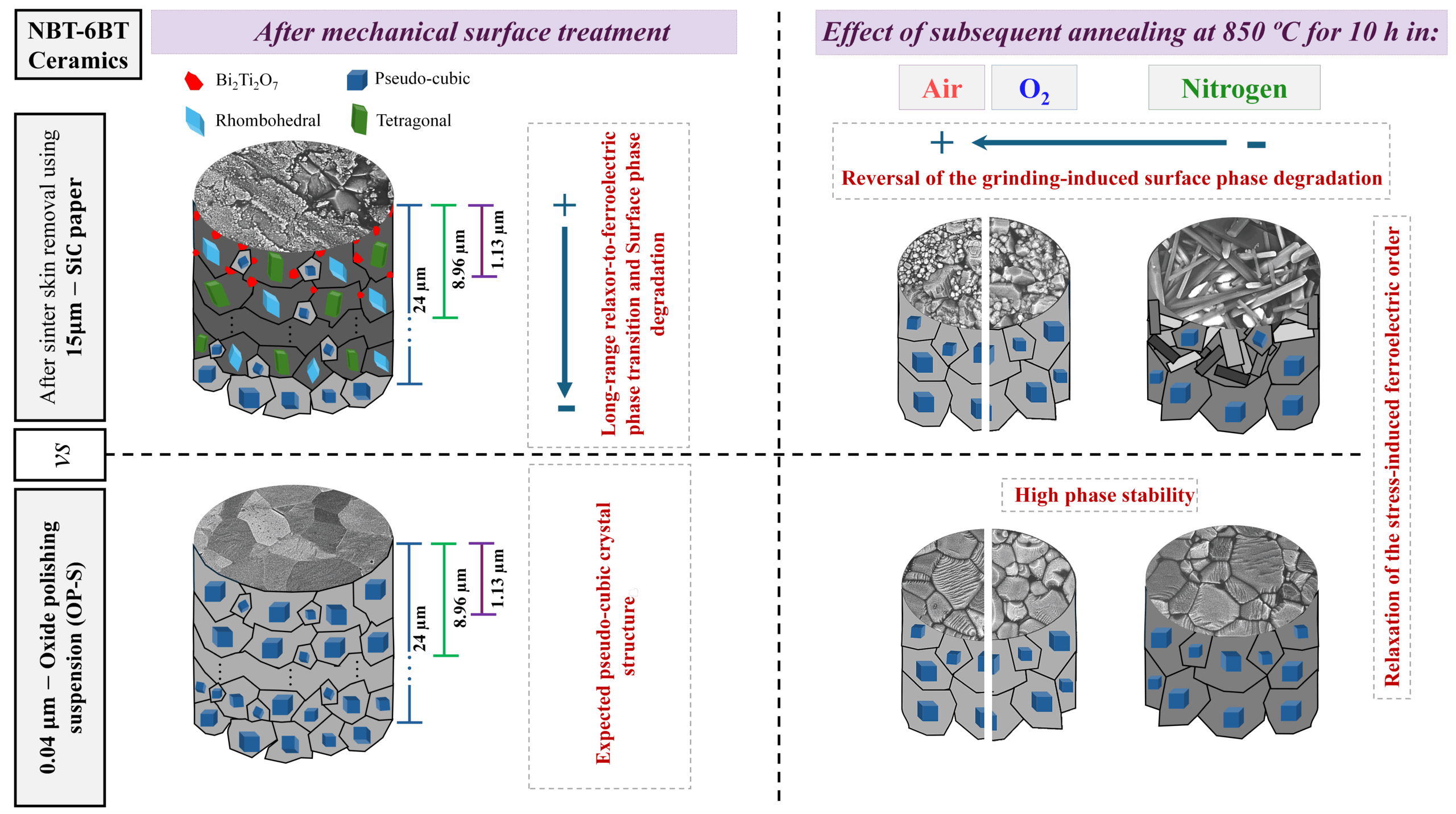
[Image above]Lithium borosilicide framework. Credit: Fässler, M. Zeilinger; TUM.
Lightweight, high energy density, and fast, reliable recharging have made lithium-ion batteries the preferred power sources for cell phones and other portable electronic devices. The basic technology is proven, but research continues to develop better materials that could allow the devices to store even more energy and improve overall battery life.
One focus has been on improved electrode materials. The graphite commonly used as the anode material in commercial batteries can degrade and has limited energy storage capacity compared with some other candidate materials. For example, scientists at the Technische Universität München (TUM; Munich, Germany) are examining a layered lithium borosilicide material with a tetrahedral structure that also features channels. The atomic-scale channels should enable the material to hold more lithium atoms than graphite, according to investigators.
“Open structures with channels offer in principle the possibility to store and release lithium atoms,” Thomas Fässler, professor at the TUM Institute of Inorganic Chemistry, said in a news release. “This is an important requirement for application as anode material for lithium-ion batteries.”
Synthesized at the Arizona State University (Phoenix) Department of Chemistry and Biochemistry, the LiBSi2 formed at 100,000 atm pressure and temperature near 900°C, according to the release. The researchers say it is stable in air and moisture and withstands temperatures to 800°C. A rendering of the material’s structure is seen in the above illustration.
Next steps for the scientists involve determining exactly how many lithium atoms the material, which they call “tum,” can store and also evaluating its expansion during charging. Results of the work so far are reported in Angewandte Chemie International Edition (DOI:10.1002/anie.201301540).

Seen in a transmission electron micrograph with overlay of chemical information, the red represents LiFePO4 while green represents iron phosphate—LiFePO4 without lithium. Credit: Sandia National Laboratory.
A more well-known electrode material for Li-ion batteries is lithium iron phosphate. A member of the naturally-occuring olivine family of minerals, LiFePO4 is safer and longer-lasting than the lithium cobalt oxide frequently used in today’s batteries for portable devices, according to scientists at Sandia National Laboratories (Livermore, Calif.).
In a news release, researchers report progress on characterizing the process by which lithium ions move in and out of LiFePO4. According to Farid El Gabaly and coworkers, a lack of understanding of this phenomenon has been a barrier to more widespread adoption of the material for Li-ion battery electrodes.
The scientists used X-ray microscopy to examine thin cross sections of the electrode from a commercial-grade battery. They mapped the locations of the lithium in about 450 particles with the battery at different states of charge, and found evidence that charging and discharging in LiFePO4 is unaffected by particle size. Instead, the process relies on a “particle-by-particle pathway … of phase transformations due to insertion of lithium ions into the cathode.”
El Gabaly said the findings contradict previous assumptions and compared the discharging process to popcorn popping. “One propagation theory said that when all the particles were exposed to lithium, they would all start discharging slowly together in a concurrent phase transformation,” he said. “We’ve now seen that the process is more like popcorn. One particle is completely discharged, then the next, and they go one-by-one like popcorn, absorbing the lithium.”
Results of the research are reported in an article in the journal Nano Letters (DOI: 10.1021/nl3031899). Authors include El Gabaly and William Chueh of Stanford University. A brief video describing Sandia’s approach to analyzing the material’s charge/discharge characteristics is also available.
Author
Jim Destfani
CTT Categories
- Basic Science
- Electronics
- Energy
- Material Innovations
- Nanomaterials