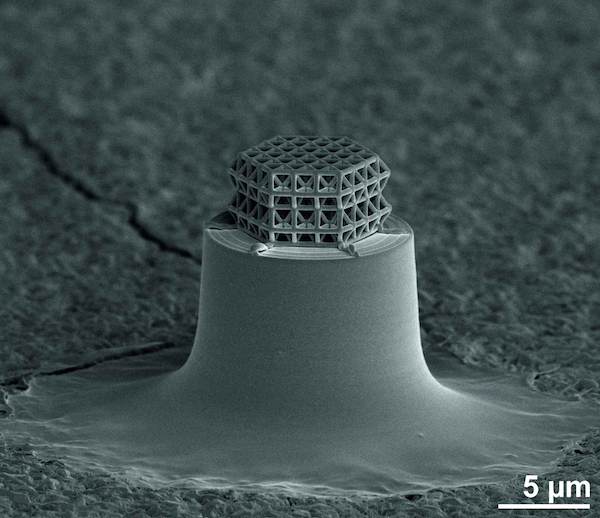
[Image above] The smallest lattice in the world is visible under the microscope only. Struts and braces are 0.2 µm in diameter. Total size of the lattice is about 10 µm. Credit: J. Bauer; KIT
Two years ago, I reported on some beautiful ceramic microlattice structures that scientists at Karlsruhe Institute of Technology had built with 3-D laser lithography. These structures had more than just looks, though—they were surprisingly strong, too.
An organized hierarchy that consists of nanoscale building blocks gives those porous structures their strength, lead author Jens Bauer told me at the time. “Because the size of the building blocks is that small, the material is much more flaw tolerant and therefore has a higher strength,” Bauer said.
So what if the size of the building blocks was even smaller—would the material be that much more flaw tolerant and that much stronger? KIT scientists set out to answer that question by one-upping their previous creation with the fabrication of smaller-yet nanolattice structures.
To create such a microscopic masterpiece, Bauer and KIT scientists again turned 3-D laser lithography to harden a polymer photoresist with a computer-controlled laser beam. While this method is great at fabricating intricate, precise, and tiny structures, it has just one small problem—it cannot go small enough.
The KIT team wanted to build nanolattice structures so small that they fall below the resolution limit of laser lithography, which can build structures with struts as small ~5–10 μm long and 1 μm wide.
So the team devised a new technique, one that seems to be inspired by Shrinky Dinks—those little plastic toys that shrink in the oven to create bite-sized crafty creations.
After fabricating microlattice structures with laser lithography, the scientists added a pyrolysis step that shrinks the lattice by 80%, resulting in über-small vitrified structures with struts shorter than 1 μm long and just 200 nm wide.
During pyrolysis, firing to ~900°C in a vacuum furnace initiates a game of chemical bond rearrangement, leaving behind only glassy carbon nanoolattices that are five-times smaller than comparable metamaterials.
So they’re small, but are they comparably strong?
“According to the results, load-bearing capacity of the lattice is very close to the theoretical limit and far above that of unstructured glassy carbon,” coauthor Oliver Kraft says in a KIT press release. “Diamond is the only solid having a higher specific stability.”
The paper’s abstract reports that the structures have material strengths of up to 3 GPa and a strength-to-density ratio that is six times higher than that of other reported microlattice structures.
“Glassy carbon is a high-technology material made of pure carbon,” the KIT press release states. “It combines glassy, ceramic properties with graphite properties and is of interest for use in electrodes of batteries or electrolysis systems.” While such strong and small porous structures could find countless other uses, some potential other applications include as filters, optical components, and insulation.
The paper, published in Nature Materials, is “Approaching theoretical strength in glassy carbon nanolattices” (DOI: 10.1038/nmat4561).
Author
April Gocha
CTT Categories
- Glass
- Manufacturing
- Material Innovations
- Nanomaterials


