[Image above] Inflammation of mice lungs after exposure to titania nanobelts. Credit: Environmental Health Perspectives; NIH.

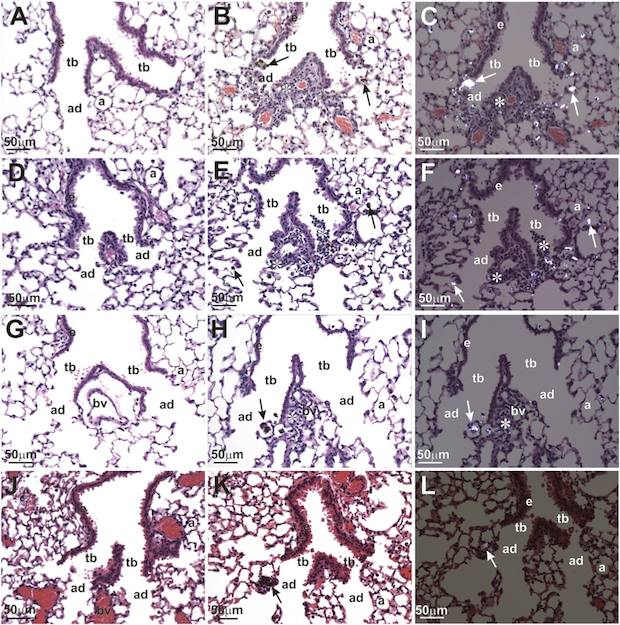
Polarized light microscopy shows inflammation of stained tissue at an alveolar duct bifurcation caused by TiO2 nanobelt exposure after one day. Nanoparticles were found within macrophages at alveolar duct bifurcations as indicated by arrows. The alveolar ducts (ad), terminal bronchioles (tb), blood vessel (bv), alveolus (a), and airway epithelium (e) are indicated. Credit: Bonner; EHP, NIH.
The “discovery” of nanomaterials about 15 years ago sparked the imaginations of researchers, and forward-thinking manufacturers followed soon thereafter. These too-small-to-see materials have properties at the nanoscale that are impossible at the bulk scale.
However, the manufacturing community and the environmental health and safety community are being justifiably cautious. As exciting as engineered nanomaterials are, the lessons learned from the asbestos, coal mining, and other industries are reminders that call for preemptive caution.
The nano-industry is burgeoning like bread dough on a hot summer day. The Project on Emerging Nanotechnologies maintains an inventory (database) of products incorporating nanomaterials. At the last update in March 2011, the inventory listed 1,317 consumer products, which represents more than 500 percent growth in the five-year period 2006–2011. United States’ manufacturers produce the largest number, followed by Europe and East Asia.
Last fall, the Centers for Disease Control and the National Institute for Occupational Safety and Health (which is part of the CDC) announced an epidemiological study to assess health risk of workers exposed to carbon nanotubes and nanofibers.
In work like this, the question quickly arises—how are results evaluated and compared? For the results to have meaning, tests must be reproducible across labs, and labs must be making apples-to-apples comparisons. That is, there needs to be consensus on what things are important to measure and how to measure those things.
A recent paper out of North Carolina State University reports on work round-robin testing of toxicology tests to evaluate pulmonary health effects of exposure to engineered nanomaterials. Led by James Bonner, NCSU associate professor of environmental and molecular toxicology, the study spanned eight institutions that used rat and mouse models to evaluate lung exposure to titania and carbon nanotubes.
In a press release, Bonner says, “The goal of creating this multicenter consortium was to have multiple labs recreate key studies using the same materials and protocols, so that policy-makers have access to consistent, comparable results from multiple institutions.”
The paper notes that, to this point, toxicity studies were difficult to compare because of a lack of standard protocols and reagents. They identified five obstacles to standardizing protocols.
- Inconsistency between batches of engineered nanomaterials;
- Inherent challenges with comparing results from different labs;
- Particle aggolomeration, which can alter toxicity;
- Method and duration of dosing, as well as dose levels;
- Handling of engineered nanomaterials prior to testing.
Besides dosage, other material factors affect toxicity, including surface characteristics, charge, shape, and size.
The labs studied titania because it is a widely used nanomaterial—in consumer products like sunscreen and in industrial products like paint. Three polymorphs were investigated: anatase–rutile nanospheres, pure anatase spheres, and anatase nanobelts. Three variants of multiwalled carbon nanotubes were studied, as well: “original” MWCNTs, purified MWCNTs (where some of the metal catalyst was removed), and MWCNTs functionalized with carboxylic acid to aid their dispersion in bodily fluids.
Both families of nanomaterials caused inflammation and inflammatory lesions in the lower portions of the rodent lungs. However, removing the metal catalyst from MWCNT made them less toxic, and so did functionalizing the surfaces with carboxylic acid. In the case of titania nanoparticles, belt-shaped particles caused more lung damage than either of the nanoparticle compositions.
“The findings are significant, but the real take-away message here is that the multicenter consortium concept works – and that means this is a starting point for assessing nanomaterials using this approach,” Bonner says in the press release. “I’m optimistic that this will serve as a blueprint for similar efforts, which will give regulators comparable data across institutions that will be easier for them to interpret.”
While policy-makers consider their next steps, manufacturers have access to helpful resources. NIOSH has a nanotechnology field test team available to work with manufacturers on a voluntary basis to identify and mitigate workplace exposure risks. The August issue of the ACerS Bulletin includes an article by a NIOSH industrial hygenist outlining the resources NIOSH offers.
Author
Eileen De Guire
CTT Categories
- Nanomaterials


