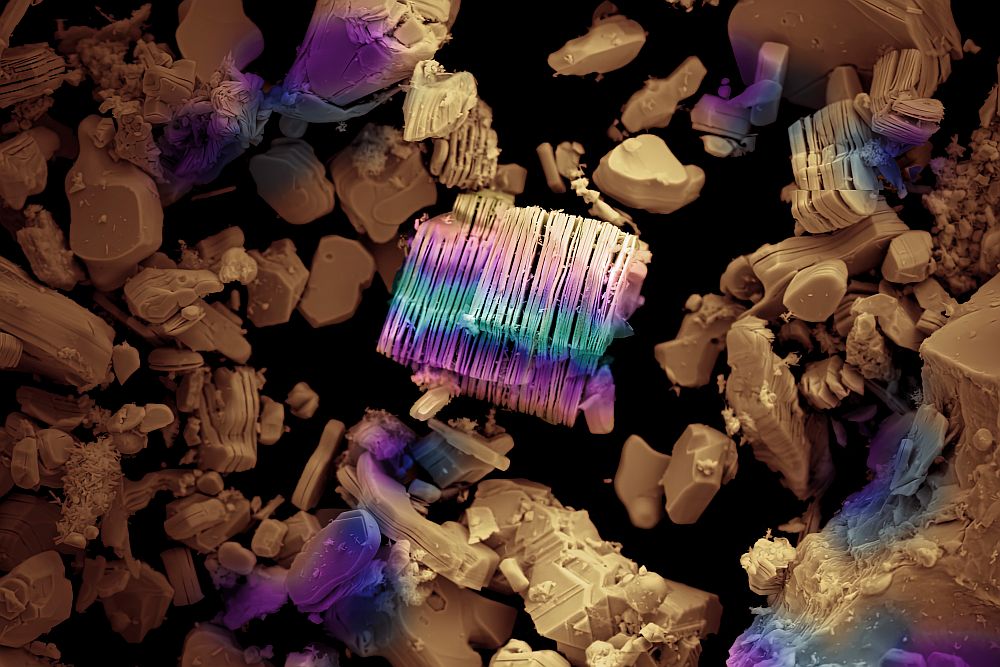
[Image above] Images of the precursor Ti3AlC2 MAX (left) used in synthesis of the 50 g Ti3C2Tx batch (right). Credit: Shuck et al., Drexel University
Drexel University researchers are at it again.
Since two groups of Drexel researchers discovered MXenes in 2011, they have conducted a ton of studies on this family of 2D transition metal carbides, carbonitrides, and nitrides. Many studies focus on the potential of these materials in various applications, including water treatment and environmental remediation.
But identifying possible applications for MXenes is only a first step toward commercialization—industry also must be able to mass produce high-quality MXenes for MXene-based devices to take off.
Researchers have investigated MXene processing techniques too, including ways to protect MXenes from oxidation. Now in the same month, the two Drexel groups each published papers describing new ways to improve processing.
Scaling production: From one to fifty grams
In the first paper published in Advanced Engineering Materials, Yury Gogotsi’s group collaborated with researchers at the Materials Research Center in Ukraine to scale production of MXenes.
For many 2D materials, challenges to large-scale synthesis “typically stem from bottom-up approaches limiting the production to the substrate size or precursor availability for chemical synthesis and/or exfoliation,” they write in the paper. MXenes are produced via a top-down synthesis approach, though, so “the process can be readily scaled with reactor volume.”
Despite the theoretical possibility to scale MXene synthesis, no studies to date demonstrate large-scale production—until this study, that is.
Using a lab-scale reactor system developed at the Materials Research Center, the researchers created large batches (50 g) of the MXene titanium carbide (Ti3C2Tx). They then used X-ray diffraction, Raman spectroscopy, and X-ray photoelectron spectroscopy to compare the composition and structure of large-batch MXenes to MXenes prepared in a small batch (1 g).
“The characterization of the two MXene batch sizes indicates that there is essentially no difference between the produced materials,” the researchers write.
In a Drexel press release, Gogotsi says usually producing nanomaterials in large quantities is only a first step toward industrialization. “…it often requires inventing completely new machinery and processes to get them in a form that can be inserted into the manufacturing process,” he says.
However, integrating MXenes into the manufacturing line is fairly easy.
“One huge benefit to MXenes is that they can be used as a powder right after synthesis or they can be dispersed in water forming stable colloidal solutions,” Gogotsi says. “Water is the least expensive and the safest solvent. And with the process that we’ve developed, we can stamp or print tens of thousands of small and thin devices, such as supercapacitors or RFID tags, from material made in one batch.”
The paper, published in Advanced Engineering Materials, is “Scalable synthesis of Ti3C2Tx MXene” (DOI: 10.1002/adem.201901241).
Water-free is guaranteed: Nonaqueous etching of MXenes
The second paper by Michel Barsoum’s group addresses a part of the synthesis process that Gogotsi mentioned briefly above—using water as the solvent.
Water is used often to dilute the etching acid during MXene chemical synthesis. And while it may be “the least expensive and the safest solvent,” it poses problems in certain applications.
“It is known that even slight presence of water in lithium or sodium ion batteries using organic electrolytes can be detrimental to their performance,” Varun Natu, first author and doctoral researcher at Drexel University, says in a Drexel press release. When used as a solvent, traces of water can be left in the MXene, which is detrimental in these battery applications.
In light of this situation, the Drexel researchers decided to develop an etching process that would not require water.
For their study, the researchers used a variety of organic solvents and ammonium dihydrogen fluoride, a chemical commonly used to etch glass. “This solution does the etching, in part because it breaks down into hydrofluoric acid, but it does not require water to dilute it or to wash away the by-products of the etching process,” the press release explains.
Not only did they show synthesis was possible with this process, they also demonstrated that electrodes made from MXenes etched in propylene carbonate resulted in sodium-ion battery anodes with nearly double the capacity to those etched in water.
“This finding opens up a huge new field of research: nonaqueous etching of MXenes,” Barsoum says in the press release. “We believe that this work will prove useful not only to the MXene community, but also to researchers throughout the field of materials science.”
The paper, published in Chem, is “2D Ti3C2Tz MXene synthesized by water-free etching of Ti3AlC2 in polar organic solvents” (DOI: 10.1016/j.chempr.2020.01.019).
Author
Lisa McDonald
CTT Categories
- Nanomaterials