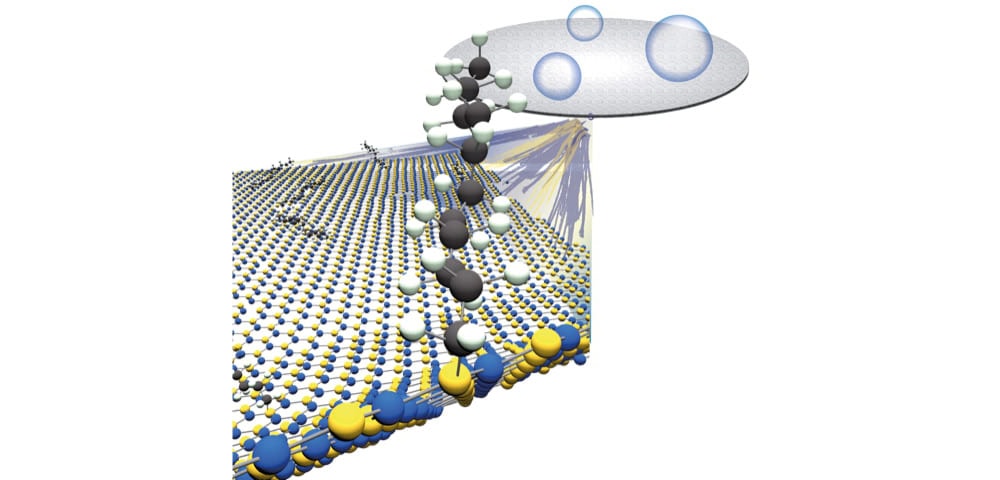
[Image above] Thanks to a protocol developed by Rice University chemists, it is now much simpler to add carbon chains to 2D hexagonal boron nitride, a material stiffer than steel and an excellent conductor of heat. Credit: Angel Martí group, Rice University
In the realm of 2D materials, hexagonal boron nitride (h-BN) holds a unique set of properties that makes it stand out from its peers.
It is reportedly four times stiffer than steel; has a thermal conductivity of 600 W/(m*K) for a single layer, which makes it a good thermal conductor; and has a wide band gap of 5.9 eV, a characteristic not found in carbon nanomaterials and makes it a good electrical insulator. These properties, in addition to pronounced chemical and thermal stabilities, make 2D h-BN an ideal additive for composites to enhance their properties.
However, as is often the case, these same properties make it difficult to manufacture functional 2D h-BN.
“…To control [h-BN’s] properties for manufacturing, you have to graft different groups onto the surface,” Angel Martí, associate professor of chemistry, bioengineering, and materials science and nanoengineering at Rice University, says in a Rice University press release.
The tight hexagonal lattice that composes h-BN, which creates its chemical and thermal stabilities, also makes h-BN highly resistant to change. This resistance makes it difficult to use h-BN as an additive because it will not bond easily with other elements. (In contrast, other layered materials like graphite can be easily modified.)
Several methods to more easily functionalize h-BN (i.e., bond it with other elements) include reactions in autoclaves and sonication-assisted and plasma-assisted methods. Yet one method—functionalization via reductive conditions—has not been sufficiently explored.
“The reduction of nanomaterials has proven to be a powerful way to modify their properties, given that reduced species present enhanced reactivity toward a variety of molecules,” Rice University chemists write in a recent paper.
The Rice University chemists, including Martí, previously experimented with using a particular reduction method, the Billups-Birch reaction, to functionalize BN nanotubes. Now, in their recent work, the chemists looked to see if that same method would functionalize 2D h-BN as well.
They mixed h-BN flakes and a carbon source (1-bromododecane) with varying amounts of lithium (Li), an alkali metal that sheds free electrons when combined with liquefied ammonia. The resulting reaction produced an alkyl radical, a chain of carbon atoms that reacted with h-BN and formed a bond.
Using Fourier-transform infrared spectroscopy and thermogravimetric analysis, the researchers determined the highest degree of functionalization is obtained when using a BN to Li molar ratio of 1:20. They then prepared films with h-BN and functionalized h-BN (fh-BN) 1:20 to compare each film’s affinity to water.
“While the angle of water in contact with the h-BN film indicates a hydrophilic surface, the angle on fh-BN is characteristic of a very hydrophobic surface,” they write in the paper. In other words, the fh-BN sheets could serve as convenient water-repelling transparent coatings.

A flake of functionalized hexagonal boron nitride created at Rice University, as seen under a transmission electron microscope. Credit: Angel Martí group, Rice University
In the press release, Martí says the modified Billups-Birch reaction process is the best method to functionalize h-BN found so far. Additionally, because the base h-BN remains stable under high temperatures, fh-BN can be returned to h-BN by simply burning off the functional chains.
In the future, the chemists plan to explore what other kinds of molecules can be grafted onto 2D h-BN. “What about benzene groups? What about ethers? What about groups that will make it compatible with other materials?” Martí says. “Ultimately, we’d like to graft different groups onto h-BN and build a library, kind of a toolbox, of functional groups that can be used with these materials.”
The paper, published in The Journal of Physical Chemistry C, is “Tunable alkylation of white graphene (hexagonal boron nitride) using reductive conditions” (DOI: 10.1021/acs.jpcc.9b05416).
Update 8/16/2019 – Hexagonal boron nitride is a good electrical, not thermal, insulator. Also, h-BN is a good thermal conductor.
Author
Lisa McDonald
CTT Categories
- Manufacturing
- Nanomaterials


