According to a press release, a team of Rice University and Lockheed Martin scientists has discovered a way to use simple silicon to increase the capacity of lithium-ion batteries by enhancing the inherent ability of silicon to absorb lithium ions
Rice University is famed for the buckyball discovery 25 years ago for nanotechnology development. The new battery work was introduced this week at Rice’s Buckyball Discovery Conference, part of a yearlong celebration of the 25th anniversary of the Nobel Prize-winning discovery.
“The anode, or negative, side of today’s batteries is made of graphite, which works. It’s everywhere,” says Michael Wong, a professor in chemical and biomolecular engineering and in chemistry. “But it’s maxed out. You can’t stuff any more lithium into graphite than we already have.”
Silicon has the highest theoretical capacity of any material for storing lithium, but there’s a serious drawback to its use. “It can sop up a lot of lithium, about 10 times more than carbon, which seems fantastic,” Wong said. “But after a couple of cycles of swelling and shrinking, it’s going to crack.”
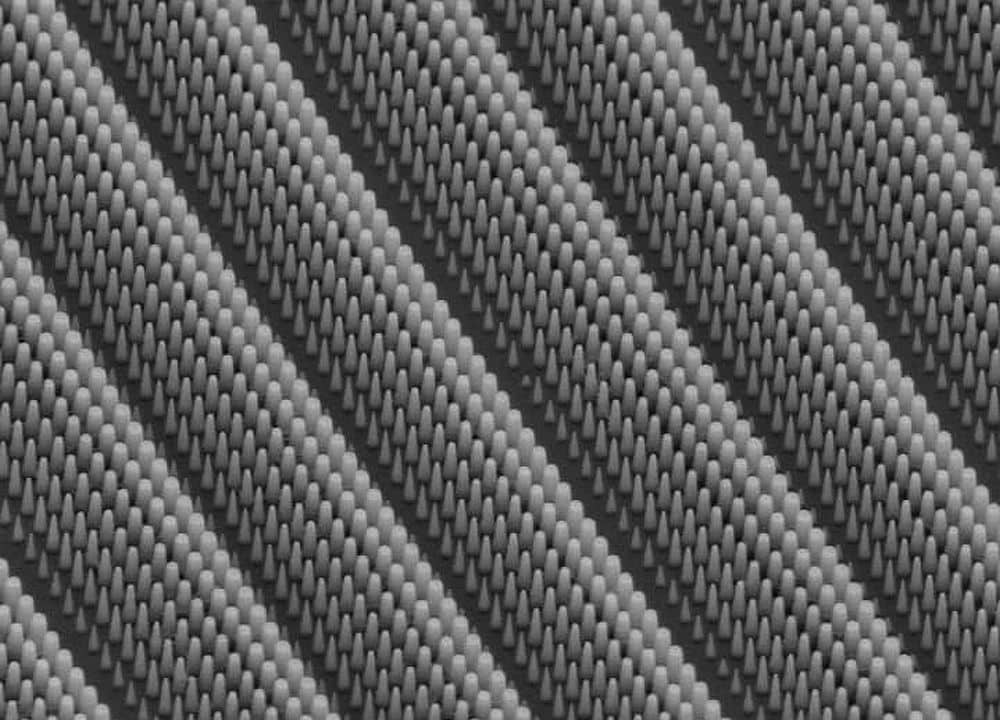
However, the researchers say they discovered that putting micron-sized pores into the surface of a silicon wafer gives the material sufficient room to expand. While common Li-ion batteries hold about 300 milliamp hours-per-gram of carbon-based anode material, the researchers determined the treated silicon could theoretically store more than 10 times that amount.
The pores, a micron wide and 10-50 microns long, form when positive and negative charge is applied to the sides of a silicon wafer, which is then bathed in a hydrofluoric solvent. “The hydrogen and fluoride atoms separate. The fluorine attacks one side of the silicon, forming the pores. They form vertically because of the positive and negative bias,” says Sibani Lisa Biswal, an assistant professor in chemical and biomolecular engineering.
Putting pores in silicon requires a real balancing act, as the more space is dedicated to the holes, the less material is available to store lithium. And if the silicon expands to the point where the pore walls touch, the material could degrade.
The researchers are confident that cheap, plentiful silicon combined with ease of manufacture could help push their idea into the mainstream.
“We are very excited about the potential of this work. This material has the potential to significantly increase the performance of lithium-ion batteries, which are used in a wide range of commercial, military and aerospace applications,” says Steven Sinsabaugh, a Lockheed Martin fellow.
CTT Categories
- Electronics
- Material Innovations
- Nanomaterials
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026