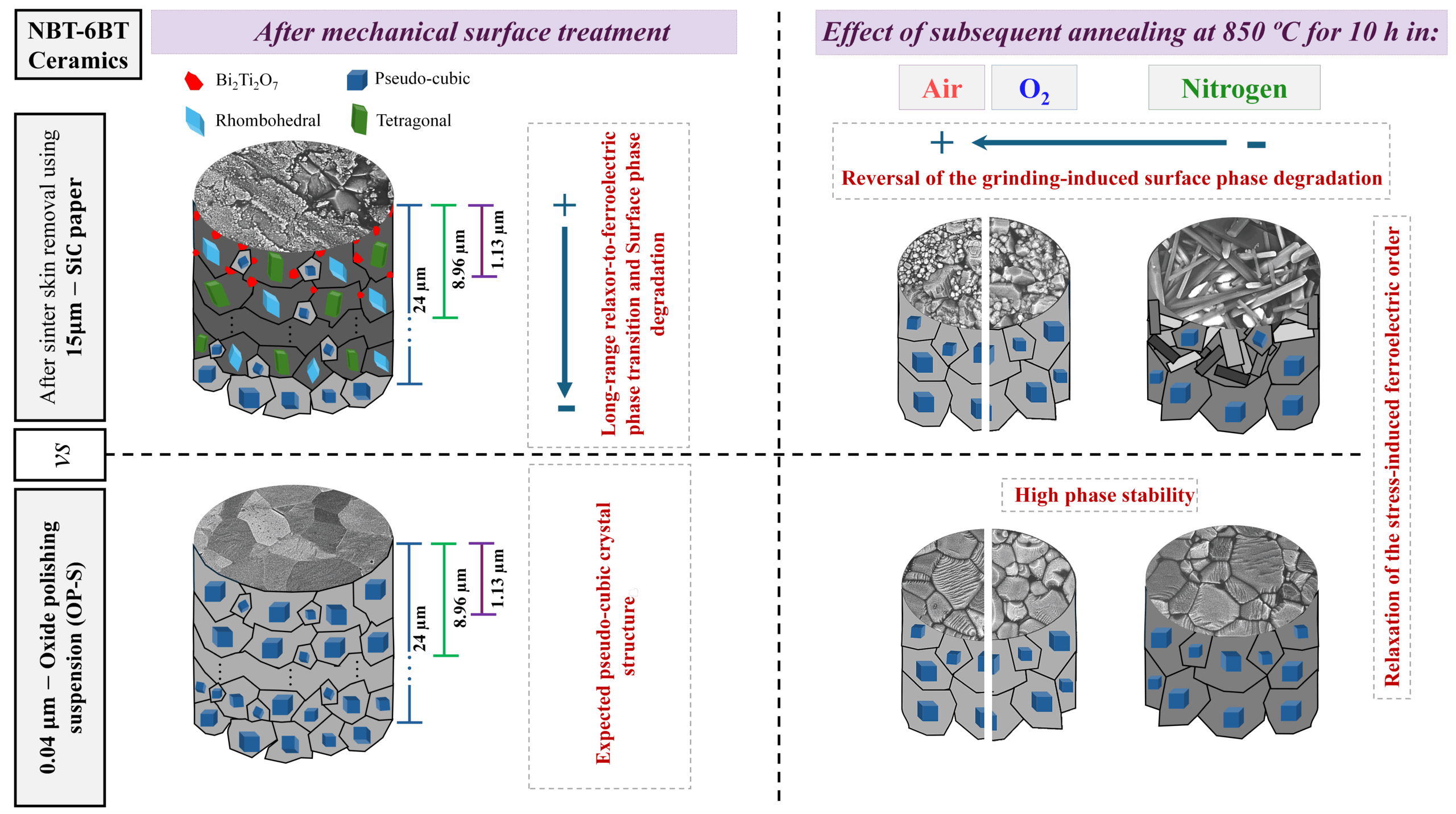
[Image above] Where did all the water on Earth come from? A new paper posits that magnesium hydrosilicates served as reservoirs of water in early Earth. Credit: Timothy M Roberts, Flickr (CC BY-SA 2.0)
It feels like my newsfeed is constantly filled with discoveries advancing space travel and our knowledge of other planets. Yet as we push our capabilities to explore the “final frontier” beyond our atmosphere, there remains so much unknown about a “final frontier” on our planet—the world ocean.
The world ocean, which is subdivided into four or five named ocean basins depending on the authority, covers about 71% of Earth’s surface. Yet more than 80% of it remains unexplored. A large reason for that is technological. Compared to the surface of the Earth, the moon, and other planets like Mars, the deep ocean is not directly illuminated by light or radio waves. So, devices used to explore areas on the surface are not directly transferrable to exploring underwater.
In recent decades, advances in technology have led scientists to realize that deep sea ecosystems are more complex than previously imagined and play an integral role in Earth’s climate system. Thus, “Our knowledge of the oceans is a key element for the future of humanity,” Audrey Azoulay, UNESCO Director-General, emphasizes when introducing a recent report by UNESCO’s Intergovernmental Oceanographic Commission on the inadequacy of funding for ocean research.
To advance ocean research and humanity into the future, however, researchers are not just looking at marine and climate ecosystems as they are today. Knowledge of how the ocean developed to this point is needed as well, yet one historical question about the ocean remains a long-standing mystery—where did Earth’s water come from?
Currently, there are two prevailing and opposing views on this matter.
- That water is primordial, i.e., water was released from inside Earth during formation.
- That water was “donated” later by water-rich aerolites (stony meteorites consisting of silicate minerals).
Scientists have traditionally favored the second explanation—that water was brought to Earth from somewhere else. However, several recent studies have increased the amount of evidence supporting the argument that Earth contained the necessary building blocks to form water on its own too.
The deuterium/hydrogen (D=H) ratio is one statistic that scientists have highlighted to support the first viewpoint. Earth’s deep mantle has a low D=H ratio quite close to that of enstatite chondrite meteorites, which are the fundamental building blocks of the young Earth. This similarity suggests that water within Earth’s interior may have come directly from the protosolar nebula.
“However, this hypothesis raises several questions. Compared with other planetary materials such as iron and silicates, water has a much lower condensation temperature and therefore would have been released to space at the high surface temperature of the newborn Earth and then by the Moon-forming impact. To avoid complete loss, water must have been stored inside the Earth in the planet’s neonatal accretion period,” researchers write in a new paper.
The researchers come from several universities in China and the Skolkovo Institute of Science and Technology in Russia. They are led by Nankai University professor Xiao Dong in partnership with Skoltech full professor Artem R. Oganov.
In their paper, they advance another hypothesis about the origins of primordial water—that it was contained in hydrous minerals.
Hydrous minerals are minerals that contain water in their structure. When certain conditions are met, such as the mineral is transported to an area with lower pressure, the minerals disassociate and release the water contained inside.
There is a sizable amount of research on the role hydrous minerals play in the water storage and transportation mechanisms within Earth’s mantle. Magnesium hydrosilicates specifically have received a lot of attention due to oxygen, magnesium, and silicon being the most abundant elements in Earth’s mantle.
However, research on magnesium hydrosilicates to date has focused on polymorphs that currently exist in Earth’s mantle—an environment that is far different from the mantle during Earth’s formative years.
For the new study, the researchers wanted to consider polymorphs that could have withstood the high temperatures and pressures of the core–mantle separation process. During that time in Earth’s history, Earth had a fairly even distribution of elements throughout, rather than the metallic core we know today. In other words, the elements that make up magnesium hydrosilicate would have been available deep within Earth millions of years ago.
To identify possible magnesium hydrosilicate polymorphs, the researchers used a variable-composition evolutionary structure prediction algorithm and ab initio molecular dynamics simulations to model the ternary system MgO─SiO2─H2O. The structure prediction algorithm was implemented using the USPEX code, which is a novel method for computational materials discovery developed by Oganov’s laboratory since 2004.
The modeling revealed two thermodynamically stable polymorphs that could have withstood the core–mantle separation process—α-Mg2SiO5H2 and β-Mg2SiO5H2 with base centered monoclinic lattices. These magnesium hydrosilicates are more than 11% water by weight and are stable at pressures of more than 2 million atmospheres.
The researchers explain that these magnesium hydrosilicates would have originally existed at the center of the forming Earth. However, as the core grew, they were displaced to shallower depths with lower pressures and disassociated into MgSiO3, MgO, and water.
“The released water would be gradually transported to the surface of the Earth, to form its hydrosphere. As to the other products of dissociation of Mg2SiO5H2, namely, MgSiO3 and MgO, they are still in the lower mantle, playing the role of its main phases,” the researchers write.
The researchers conclude the paper by highlighting some of the numerous implications this hypothesis could have on other areas of research. For example, it could explain why Mars is so dry compared to Earth.
“Mars, for example, is too small to produce pressures necessary to stabilize magnesium hydrosilicate,” Oganov says in a Skoltech press release. “This explains why it is so dry and means that whatever water exists on Mars, it likely came from comets.”
Dong adds the hypothesis could also improve our understanding of planets outside our solar system.
“To be habitable, an exoplanet has to have a stable climate, which requires both continents and oceans. So there has to be water, but not too much,” he says in the press release. “There was an estimate that for an Earth-like planet of any size to be habitable, it should have no more than 0.2% water by weight. Our results imply that for large Earth-like planets, called ‘super-Earths,’ the story is likely different: In such planets, pressures stabilizing the magnesium hydrosilicate must exist even outside the core, locking up large amounts of water indefinitely. As a result, super-Earths can have a much greater water content and still support the existence of exposed continents.”
Finally, Oganov notes that the hypothesis could have implications for a planet’s magnetosphere. “At temperatures of more than 2,000 degrees Celsius, magnesium hydrosilicate will conduct electricity, with hydrogen protons serving as charge carriers. This means that our hydrosilicate will contribute to the magnetic fields of super-Earths,” he says in the press release.
In an email, Oganov says that if the new hypothesis is correct, then the water released by the magnesium hydrosilicates could have brought other components with it to Earth’s surface, which would imply there are additional detectable geochemical signatures of the process.
“But it will require us to understand better the chemical behavior of the elements at ultrahigh pressures. We are exploring this,” he says.
The paper, published in Physical Review Letters, is “Ultrahigh-pressure magnesium hydrosilicates as reservoirs of water in early Earth” (DOI: 10.1103/PhysRevLett.128.035703).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Environment
- Modeling & Simulation