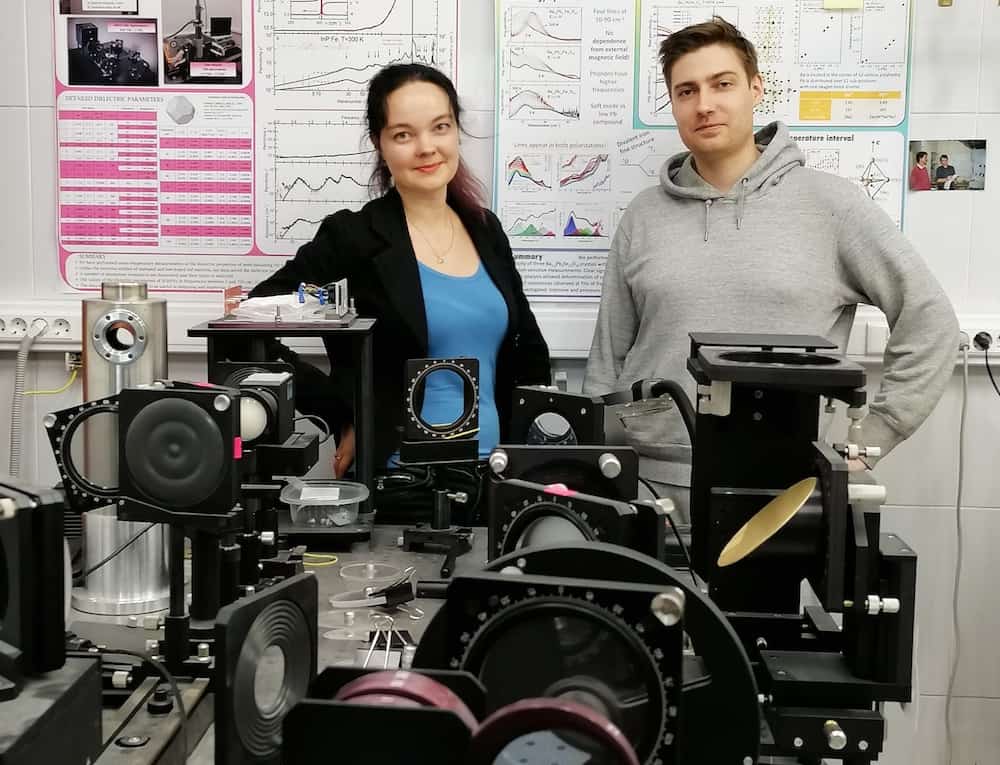
[Image above] MIPT senior researcher Dr. Liudmila Alyabyeva and Moscow State University Ph.D. student Evgeny Gorbachev at the MIPT Laboratory of Terahertz Spectroscopy, where they and colleagues performed testing on epsilon iron oxide (ε-Fe2O3) nanoparticles. Credit: Moscow Institute of Physics and Technology
When we distinguish between types of iron oxide, we often focus on the number of iron and oxygen atoms coordinated with each other—hematite (Fe2O3), magnetite (Fe3O4), wüstite (FeO).
However, the way in which iron and oxygen atoms coordinate with each other is important to consider as well. For example, hematite is the crystal structure of iron(III) oxide known as alpha phase (α-Fe2O3). Other structures include the stable and common gamma phase called maghemite (γ-Fe2O3), plus more exotic modifications like the epsilon (ε-Fe2O3) and beta (β-Fe2O3) phases.

Examples of various iron(III) oxide crystal structures. Credit: Evgeny Gorbachev, Moscow State University
The various phases of Fe2O3 can exhibit different material properties. For example, while hematite is nonmagnetic, maghemite is ferrimagnetic and is used as media for magnetic recording.
The epsilon phase of Fe2O3 is of interest to scientists for several reasons. One, this phase has an extremely high ability to resist an external magnetic field, a property called coercive force. Its value reaches 20 kOe at room temperature, which is comparable to the parameters of magnets based on expensive rare-earth elements. Two, ε-Fe2O3 absorbs electromagnetic radiation in the sub-terahertz frequency range (100–300 GHz) through the effect of natural (i.e., in zero magnetic field) ferromagnetic resonance.
The second point is particularly exciting for next-generation communication technologies, as sixth generation (6G) wireless technologies expect to use the sub-terahertz range as a working range.
Despite these desirable properties, ε-Fe2O3 has not yet found industrial application due to difficulties obtaining the rare phase. The common synthesis approach—stabilization in a silicon dioxide matrix—produces only small quantities, may require toxic surfactants, and can take up to a month to complete.
Researchers have investigated several silica-free routes to produce ε-Fe2O3, such as hydrothermal, plasma dynamic, and microwave torch discharge methods, but these approaches have resulted in ε-Fe2O3 with a high fraction of impurities. So, other researchers are focusing their efforts on improving silica-based methods instead.
In a recent paper, a group of materials scientists in Russia refined a silica-based method for synthesizing ε-Fe2O3, which was inspired by classical work on the synthesis of epsilon-iron oxide nanoparticles published in 2004.
“After reading this work, we decided that the approach implemented in this work was very nonoptimized. Furthermore, a bunch of the following publications used the same technique. So, our goal became to make the process much faster,” Evgeny Gorbachev, Ph.D. student in the Department of Materials Sciences at Moscow State University and first author of the work, explains in an email.
The approach involves preparing an iron(III) nitrate water–alcohol solution and adding tetraethoxysilane. The solution is mixed to obtain a gel, which is dried overnight, and then it undergoes a thermal treatment before the iron oxide nanoparticles are washed from the silica matrix using an aqueous solution of sodium hydroxide.
Gorbachev says they played with the hydrolysis condition of the tetraethoxysilane and shortened the time required for the process immensely—from 24 days to just 2 hours—by increasing the hydrolysis temperature up to 60°C (140°F).
X-ray diffraction phase analysis and transmission electron microscopy confirmed a high yield of ε-Fe2O3 with high magnetic coercivity in the recovered iron oxide nanoparticles. In particular, ε-Fe2O3 nanoparticles smaller than 20 nm exhibited a coercivity of 0.5–6.5 kOe, which allows use of the material as magnetic recording media. Thus, “there is no need for an artificial decrease in the coercivity of epsilon iron oxide, for example, by doping the material with gallium, cobalt and titanium ions as implied in ref. 11,” they write.
Gorbachev says they are now working on developing a fabrication method for hard-magnetic ceramics based on epsilon iron oxide nanoparticles, which “will be able to be applied in industry as a very durable rare-earth-free permanent magnet and a media for absorption of electromagnetic radiation in the 6G working range.”
In a Moscow Institute of Physics and Technology press release, MIPT Laboratory of Terahertz Spectroscopy senior researcher Dr. Liudmila Alyabyeva says they are “happy” to share their research with communication engineers and “look forward to being able to hold a 6G phone in our hands.”
The paper, published in Journal of Materials Chemistry C, is “Tuning the particle size, natural ferromagnetic resonance frequency and magnetic properties of ε-Fe2O3 nanoparticles prepared by a rapid sol–gel method,” (DOI: 10.1039/D1TC01242H).
Author
Lisa McDonald
CTT Categories
- Material Innovations
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026