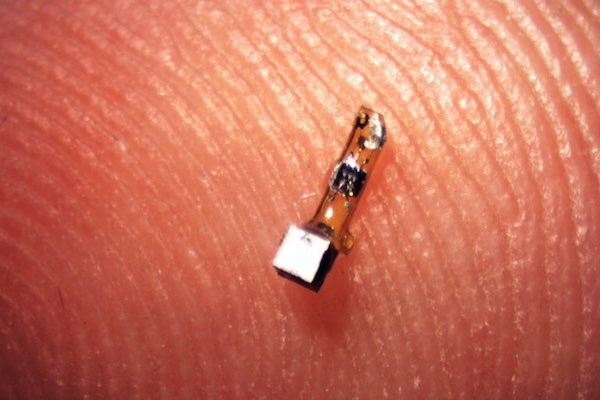
[Image above] A newly developed sensor mote contains a piezoelectric crystal (silver cube) plus a simple electronic circuit and is powered by ultrasound pulses produced by a transducer outside the body. Credit: Ryan Neely; UC Berkeley
I long for the day when in-body sensors can give me a real-time readout of precisely what’s happening in my body—quantifications of physiological parameters that can tell me when to eat a banana, drink more water, or take an extra vitamin supplement.
Perhaps even more pertinent than my desire for more personal data, however, are onboard sensors that can mediate medical conditions by monitoring the function of internal organs or detecting neural activity, for example.
Beyond just monitoring, the hope is that such sensors eventually could stimulate nerves and muscles with electrical signals, a field of biomedical electronics called “electroceuticals.” In situations of nerve damage of muscle dysfunction, these sensors could act as the missing link between the brain and the body.
Researchers at the University of California, Berkeley have now made a big step towards that vision—they have developed a new type of next-gen implantable biomedical sensor that uses ultrasound to wirelessly communicate within the body.
The sand grain-sized implantable sensors—which the researchers call neural dust motes—harness the power of piezoelectric crystals to record the electrical activity of muscles and nerves. Ultrasound both powers the sensors and serves as their readout.

Diagram showing the components of the sensor. The entire device is covered in a biocompatible gel. Credit: UC Berkeley
The sensors’ piezoelectric crystals vibrate with ultrasound waves, generating electricity to power a tiny transistor aboard the mote. By harnessing the power of piezoelectricity, the sensors require no batteries, wires, or power packs.
Bodily changes in electricity—when a nerve cell fires, for example—affect how the crystal vibrates, altering the readout signal of the sensor, or backscatter, which can be detected outside the body.
Using rats, the team has so far shown that the sensor can report electrical activity from peripheral nerves and muscles, but the proof-of-concept has bigger implications.

The sensor attached to a nerve fiber in a rat. Credit: Ryan Neely; UC Berkeley
“I think the long-term prospects for neural dust are not only within nerves and the brain, but much broader,“ Michel Maharbiz, an associate professor of electrical engineering and computer sciences and one of the study’s two main authors, says in a UC Berkeley press release. “Having access to in-body telemetry has never been possible because there has been no way to put something supertiny superdeep. But now I can take a speck of nothing and park it next to a nerve or organ, your GI tract or a muscle, and read out the data.”
The small sensors are currently 3 mm by 1 mm by 4/5 mm, but the researchers are working to shrink them even smaller—a requirement for integration with the central nervous system, which also would open the door to autonomous control of prosthetics.
And to open the technology to even more interesting applications, the team is reportedly working to develop the sensors with biocompatible films instead of the surgical grade epoxy they’re currently using to coat the motes.
“The vision is to implant these neural dust motes anywhere in the body, and have a patch over the implanted site send ultrasonic waves to wake up and receive necessary information from the motes for the desired therapy you want,” graduate student Dongjin Seo says in the release. “Eventually you would use multiple implants and one patch that would ping each implant individually, or all simultaneously.”
Watch this UC Berkeley video to see more about this stimulating development.

Credit: UC Berkeley; YouTube
The paper, published in Neuron, is “Wireless recording in the peripheral nervous system with ultrasonic neural dust” (DOI: 10.1016/j.neuron.2016.06.034).
Interested in implantable biomedical materials? Check this out, too: Another group of researchers recently devised silicon nanoparticles that can be activated by light to stimulate nerve and muscle cells.
Author
April Gocha
CTT Categories
- Biomaterials & Medical
- Electronics
- Material Innovations


