[Image above] If small enough, zirconia rods exhibit shape memory and superelastic properties without crumbling. Credit: Schuh; MIT.
Zirconia, one of the workhorses of the ceramic stable, has a well-known flaw—the crystallographic transition at about 1,150˚C from tetragonal to monoclinic structure involves a large volume change, and the accompanying strains can leave you with a pile of dust instead of a working ceramic. Adding yttria, magnesia, or other dopant stabilizes the tetragonal (or cubic) structure and avoids the structural change, rendering the material useful at high temperature.
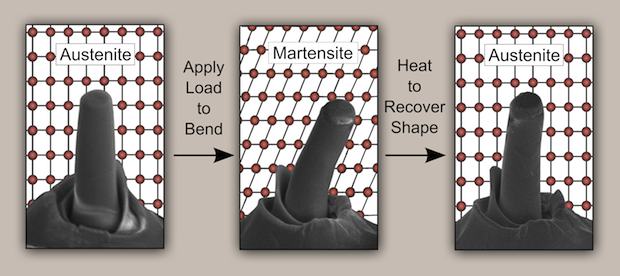
The tetragonal-to-monoclinic structure transition corresponds to an austenite-to-martensite transformation. Metallurgists will recognize the connection to the austenite-martensite-austenite transformation that characterizes shape memory alloys and superelasticity. A group led by MIT professor Christopher Schuh recently demonstrated superelasticity and the shape memory effect in zirconia, according to a new article in Science.
Superelasticity is a stress-strain response corresponding to the tetragonal-monoclinic-tetragonal transformation that is fully reversible and independent of temperature. Shape memory is a function of temperature—the tetragonal crystal structure (and material “shape”) is restored by applying heat to the monoclinic structure material. For both, the stress–strain curve is a hysteresis loop.
The MIT group realized that in zirconia, the tetragonal-monoclinic transformation is so destructive because of the strains imposed on grains by neighboring grains also undergoing volume expansions, not the volume expansion itself. That is, any given grain wants to change its volume to accommodate the new structure, but its pesky neighbors are expanding at the same time and trying to fill the same space. In zirconia, this “strain mismatch” can lead to shear strains of up 15% and inevitable fracturing.
Schuh’s group got around the problem by making “oligocrystals”—very small samples with very few grain boundaries and high surface area to volume ratio. The high surface area aids with stress relaxation. Minimizing interfaces minimizes competition between grains for space.
The group had the advantage over previous researchers because processes now exist to make very small test samples, and they ion-milled micropillars of zirconia out of larger polycrystalline samples. (Samples were doped with either ceria, yttria, or both.) The trick was to machine samples that were smaller than the average grain size of the bulk material so that the surface area was much greater than the grain boundary area, according to the paper. The effective diameter of the pillars was in the 1–2 µm range. Based on the image above, they appear to be 4–5 µm tall. Samples were subjected to compressive stress and bend stress.
The zirconia micropillars withstood a remarkable 7% strain. For comparision, NiTi, the most-used shape memory alloy, accommodates strains up to 8%. According to the press release, most ceramics break when bend loads exceed 1%. In an accompanying Perspectives article in Science, Northwestern University professor Katherine Faber notes that an aluminum can subject to 7% strain would “crumple with no hope of recovery.”
This work opens a pathway for fabricating devices such as actuators and switches for high-temperature or corrosive environments. Other applications include energy harvesting, drug delivery devices, and perhaps some dreamed-of applications such as self-healing in composites.
Understanding the influence of size effects could lead to the discovery of ductility in other ceramic materials. Faber suggests, for example, that some lanthanide compounds and silicate compounds could exhibit superelasticity if engineered properly.
Author
Eileen De Guire
CTT Categories
- Basic Science
- Material Innovations


