[Image above] Structure of the world’s first molecule with a triple bond between boron and carbon. Credit: Rian Dewhurst, Julius Maximilian University of Würzburg
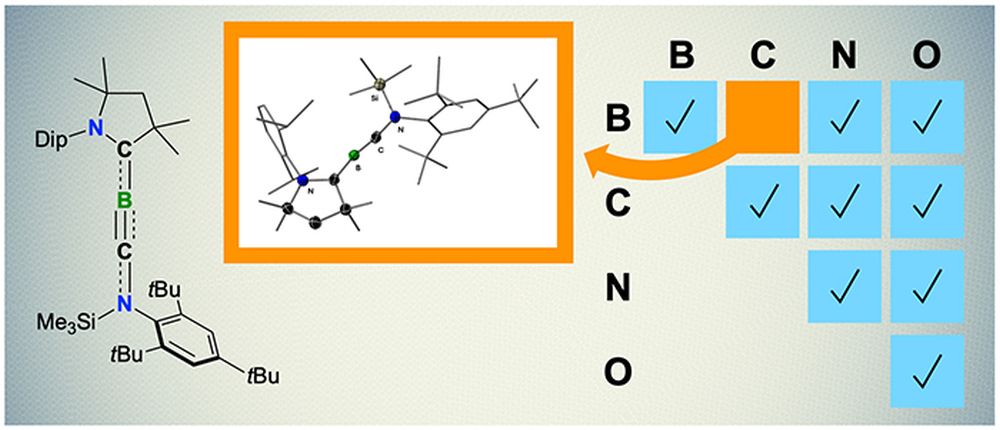
Sharing is caring, and this sentiment is exemplified by the iconic quartet of boron, carbon, nitrogen, and oxygen. These four elements are the core of ceramic compounds, and their similar electronic properties allow them to form stable double and triple bonds with each other (and with themselves).
But there is a noticeable exception to this rule: boron and carbon. This pairing is the only combination of the four elements that has not successfully formed a stable triple bond with each other; only simple transient molecules featuring boron–carbon triple bonds have been experimentally confirmed.
However, if transient examples exist, that suggests a stable bond could be achieved under the right conditions, as demonstrated in a previous CTT. In a new paper, researchers from Julius Maximilian University of Würzburg successfully achieved the necessary conditions to close this gap in the family of first-row p-block compounds with triple bonds.
To create the so-called neutral “boryne” (named as such by extension from the term alkyne), the researchers performed a multistep synthesis process starting with bromoborylene, which underwent reductive dehalogenation, isolation of the dimeric dipotassium complex, and then addition of trimethylsilylchloride. This process resulted in an orange solid (the boryne) that was stable indefinitely under argon at room temperature but decomposed in solution at room temperature.
The researchers synthesized several derivates of the boryne in solution, as well as performed quantum chemical calculations, to confirm the boryne’s geometry. These experiments revealed the triple bond comprised one σ and two orthogonal π bonds, which is the usual structure for triple bonds.
The researchers write that their study is “a testament to the power of iterative synthetic variation of the substituents in low-valent main-group species,” which in this case provided the right balance of steric and electronic factors to “allow the isolation of the title compound as a metastable species en route to a more thermodynamically stable borylene species.”
In a press release, first author Maximilian Michel, the doctoral student who made the molecule in the laboratory, notes that boryne’s geometry is very uncomfortable for boron atoms, as it puts them in a linear arrangement with the carbon atoms. However, “Compounds in which individual atoms feel ‘uncomfortable’ often show a very interesting reactivity,” he says, and this potential reactivity is what the researchers are now investigating.
The paper, published in Nature Synthesis, is “The synthesis of a neutral boryne” (DOI: 10.1038/s44160-025-00763-1).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Material Innovations


