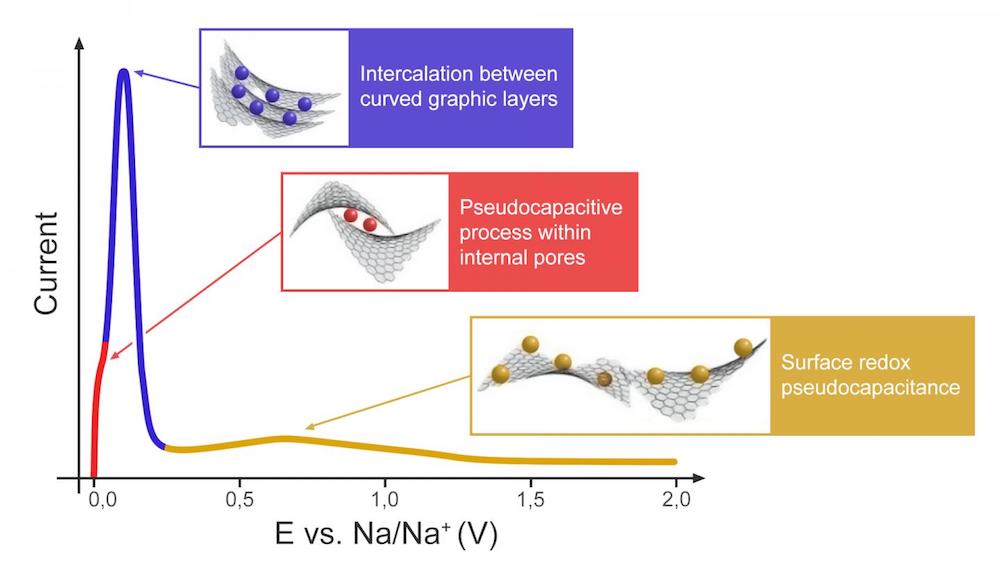
[Image above] Illustration of the proposed three-stage charge storage mechanism for hard carbon anodes. Credit: Bobyleva et al., Electrochimica Acta
When it comes to energy storage technologies, we’ve talked quite a bit about lithium-ion batteries on CTT this year.
Li-ion batteries are the most popular electrochemical power sources used today. They power a wide variety of electronic gadgets, from smartphones and laptops to power tools and medical equipment, and they serve as the heart of current widely commercialized electric vehicles.
However, the cost to produce Li-ion batteries is expected to grow in the coming years due to a key element found in many Li-ion batteries—cobalt.
Cobalt is used in the cathode of many Li-ion batteries to ensure structural stability during charge/discharge cycling. The metal is expensive, both monetarily and in terms of associated human costs, and the increasing demand for electric vehicles—in addition to increased calls for ethical mining of the metal—are expected to result in shortages of cobalt, driving up costs in the long term. (In the short term, costs are at an all-time low due to overexuberant projections and COVID-19.)
Researchers are investigating alternative high-energy-density battery technologies that do not require cobalt, such as lithium-sulfur batteries or Li-ion batteries with cobalt-free cathodes. As you can tell from the examples, many of these technologies still involve lithium. However, another non-lithium alternative being investigated is sodium-ion (Na-ion) batteries.
Na-ion batteries are analogous to Li-ion batteries, but instead of lithium ions moving between the cathode and anode, sodium ions do so instead. The two batteries have similar electrochemical characteristics, but Na-ion batteries cost less to produce because Na-ion batteries do not require cobalt in the cathode and sodium is more abundant than lithium.
However, there is a key difference between Na-ion and Li-ion batteries. Sodium ions are much bigger than lithium ions, so the material typically used for the anode in Li-ion batteries—graphite—cannot be used for Na-ion batteries because the sodium ions cannot intercalate, i.e., be inserted, into the graphite structure.
Instead, hard carbon is the material most often used for the anode in Na-ion batteries.
Hard carbon refers to carbons arranged in such a way that they are nongraphitizable, i.e., they cannot be cleanly separated into thin, separate layers of graphite. The disordered structure provides spaces large enough for sodium ions to intercalate.
Intercalation is not the only way charge is stored in anodes, however. Another important charge storage mechanism to consider is adsorption.
Adsorption refers to adhesion of ions on the surface of the anode material. (In contrast to intercalation, which refers to insertion of ions within the bulk.) Compared to batteries in which intercalation is the main charge storage mechanism, batteries that store charge through adsorption (known as supercapacitors) demonstrate low specific energy density and high self-discharge rate.
In Li-ion batteries, intercalation is the main charge storage mechanism in graphite anodes. But in Na-ion batteries, the relationship between intercalation and adsorption in hard carbon anodes remains an open question.
In an email, Oleg Drozhzhin, senior research scientist at the Skoltech Center for Energy Science and Technology and Lomonosov Moscow State University, explains several of the main views currently held on hard carbon charge storage mechanisms.
“The most popular and old one is ‘intercalation-adsorption’ (i.e., intercalation between curved graphitic layers within the sloping voltage region and adsorption at low-voltage plateau),” he says. “Another model, named ‘adsorption-intercalation,’ supposed Na+ adsorption at the surface or defects sites with further intercalation within the low-voltage plateau.”
Several alternative models—such as “adsorption-filling” and three- or four-stage models—also exist, but Drozhzhin says determining which model is correct is difficult.
“…hard carbon is an extremely complex object for studying. As it does not possess long-range ordering, usual methods such as X-ray diffraction or transmission microscopy could not reveal the type of electrochemical process,” he says.
Drozhzhin says several sophisticated methods have provided some insight into the mechanisms, such as the work of Clare Grey’s group at the University of Cambridge using solid-state nuclear magnetic resonance, but “at the moment there is no method that could definitely determine the charge storage mechanism in hard carbon.” As such, “The existence of several opinions on a complex subject is the best way to understand the nature of things,” he says.
Based on this premise, Drozhzhin and colleagues at Lomonosov Moscow State University, Skolkovo Institute of Science and Technology, and Tokyo University of Science decided to conduct their own study on the charge storage mechanisms in hard carbon. And in the recently published paper on their research, they propose a model that differs from the currently most popular view.
In their study, they used linear sweep voltammetry to analyze the pseudocapacitive behavior of hard carbon. (Pseudocapacitance refers to adsorption with certain chemical interactions allowing charge transfer.) And based on their findings, they agree that a three-stage “adsorption-intercalation-adsorption” process first suggested by Bommier et al. in 2015 is a good model for interaction between sodium ions and the hard carbon anode.
“First, pseudocapacitive-type reaction between Na+ and carbon open surface occurs at high potentials,” they write in the paper. “After the surface is filled, intercalation of Na+ between pseudographitic layers takes place, which is evidenced by low b-values (close to 0.5).”
“Finally, another type of pseudocapacitive-type process is observed at very low (~20 mV) potentials. … Under the theory of open and internal porosity, this third process can be considered as pseudocapacitive Na+ filling within internal pores, since this type of electrochemical reaction may occur only after filling the bulk volume of the material with Na+,” they conclude.
In the future, Drozhzhin says there are several aspects of anode materials for Na-ion batteries they wish to explore further, including
- Safety issues, particularly the process of sodium dendrite formation during long-term cycling;
- Self-discharge, particularly the rate of charge loss at different states of charge; and
- Full cells, particularly which cathode materials combine best with hard carbon anodes.
The paper, published in Electrochimica Acta, is “Unveiling pseudocapacitive behavior of hard carbon anode materials for sodium-ion batteries” (DOI: 10.1016/j.electacta.2020.136647).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Energy