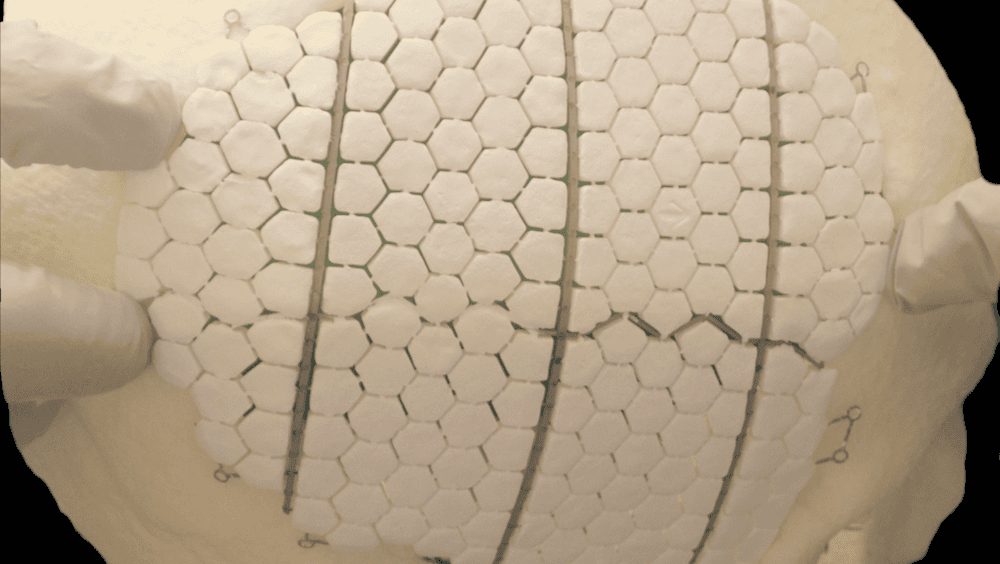
[Image above] Researchers in Sweden developed this experimental bioceramic implant, which is composed of calcium phosphate tiles reinforced and interconnected by an additively manufactured titanium frame. Credit: Omar et al., Proceedings of the National Academy of Sciences (CC BY-NC-ND 4.0)
In just over one month, the December issue of the Bulletin will be landing at your door. And in line with past years, the December issue will highlight the role of ceramic and glass materials in our everyday lives.
This year, the focus of the December issue is ceramic and glass materials in the human body. In anticipation of this issue, today’s CTT looks at one area of medicine in which ceramics are poised to play a big role—cranioplasty.
Cranioplasty refers to surgical reconstruction of a defect in the skull to restore continuity and provide support for the overlying soft tissues. The practice is a very old one—there is strong evidence Incan surgeons were performing rudimentary cranioplasty in the 15th century—but the technique only became common during the second half of the 20th century, due largely to warfare providing an impetus to improve our ability to cover large cranial defects.
To date, autologous bone grafts, or grafts made from bone obtained from other areas of the patient, are the standard for reconstructive treatment. Yet this approach is associated with frequent complications, in particular relatively high resorption, protrusion, and infection rates and a high rate of donor-site morbidities.
In the past few decades, researchers have extensively investigated alloplastic materials, or synthetic materials that substitute for tissue, as another option for cranioplasty grafts. Much research on a variety of metals, ceramics, plastics, and resorbable polymers over the years significantly advanced use of alloplastic grafts, but these materials come with their fair share of complications as well. Thus, the search for an ideal material for cranioplasty grafts continues.
Calcium phosphate ceramics are one group of materials that have played a central role in modern alloplastic cranioplasty research due to their biocompatibility and osteoconductivity, i.e., the ability of bone-forming cells in the grafting area to move across a scaffold and slowly replace it with new bone.
Calcium phosphate cements in particular have gained an edge over granular calcium phosphates because of advantages afforded by the cements’ self-hardening properties, which make molding the brittle ceramic into a desired shape easier. Often, the cements are combined with or overlaid on other materials such as bioresorbable fibers or titanium mesh, respectively, to augment strength of the graft.
In recent years, several studies have shown calcium phosphate ceramics that consist of several phases, such as beta-tricalcium phosphate (β-TCP) and hydroxyapatite, exhibit improved or new properties compared to ceramics with a single phase. For example, high protein adsorption and osteoinduction, or the ability to stimulate cells to change into bone-forming cells. More researchers are now exploring mixed-phase calcium phosphate ceramics, such as one collaborative group of researchers in Sweden.
Repairing large cranial defects: Bioceramic implant stimulates regeneration of bone
In a recent open-access study, researchers from the University of Gothenburg, Uppsala University, and Karolinska University Hospital and Karolinska Institutet looked to create a synthetic ceramic implant that could regenerate bone in large cranial defects, an area of research that “has thus far attracted limited attention.”
The researchers chose a powder mixture of β-TCP/dicalcium pyrophosphate and monocalcium phosphate monohydrate for their ceramic, which they mixed with glycerol to form a paste. They molded this bioceramic paste in the form of hexagonal tiles around an additively manufactured titanium frame and then left it to set overnight in sterile water, a process that also eliminated the glycerol.
The titanium-reinforced bioceramic implant and a control implant made only of titanium were placed in sheep skulls for testing. Following analysis of observations recorded at three months and 12 months, the researchers drew several notable conclusions.

Phase composition of the bioceramic: anhydrous dicalcium phosphate (84.74%), β-TCP (8.34%), dicalcium pyrophosphate (6.77%), hydroxyapatite (0.11%), and brushite (0.04%). Credit: Omar et al., Proceedings of the National Academy of Sciences (CC BY-NC-ND 4.0)
Soft tissue adaption
The bioceramic implants revealed defect restoration and soft tissue adaptation in sheep cranial defects. In contrast, soft tissue contraction was apparent around titanium implants, with visible metal on the skin and dura sides.
Bone growth
In the sheep skull, the bioceramic implant promoted a higher degree of bone formation, remodeling, and osseointegration compared to the titanium implant, leading to enhanced repair of the cranial defect. Outside the skeletal envelope, only the bioceramic implant promoted bone formation and maintained bone. Regardless of the location, the regenerated bone from the bioceramic had a composition similar to that of the native bone.

Scoring of bone growth in different regions of interest. All animals with bioceramic implants had bone, whereas bone was never detected in animals with titanium implants. Scoring legend: −, no bone detected in any of the 12 rectangles; +, bone detected in 1–4 out of 12 rectangles; ++, bone detected in 5–8 out of 12 rectangles; and +++, bone detected in 9–12 out of 12 rectangles. Statistically significant differences are indicated by a hash sign and asterisks. Credit: Omar et al., Proceedings of the National Academy of Sciences (CC BY-NC-ND 4.0)
In the discussion section, the researchers note two main limitations of the study: the absence of a mechanical evaluation after bone regeneration, and the absence of cellular and molecular techniques to shed light on the underlying ceramic-to-bone transformation mechanisms. Despite these limitations, the researchers say the study provided proof-of-concept for this bioceramic’s potential to promote in situ bone regeneration and osseointegration.
“We believe this principle will compete with existing treatment principles of bone transplantation, and plastic and metal implants,” Peter Thomsen, senior author and professor at University of Gothenburg, says in the press release.
The open-access paper, published in Proceedings of the National Academy of Sciences, is “In situ bone regeneration of large cranial defects using synthetic ceramic implants with a tailored composition and design” (DOI: 10.1073/pnas.2007635117).
Author
Lisa McDonald
CTT Categories
- Biomaterials & Medical
- Material Innovations


