[Image above] Credit: Science Magazine; YouTube
I have never seen a hummingbird whose colors look dull, drab, and faded out, as if the flitting critter has been left out in the sun too long—those iridescent feathers are always bright and shimmering as if they were brand new.
Which got me wondering: why don’t the vibrant colors found in nature ever fade?
After all, those hues are subjected to the same powerful UV radiation that causes our manmade paints and dyes to break down and get drab with time. But shimmering butterfly wings, brightly colored beetle shells, and peacock plumes don’t go dull.

Credit: David Denicolò; Flickr CC BY-NC-ND 2.0
Well, it turns out that not all colors are created equal.
Most of the colors we humans generate for paints, pigments, and dyes are so-called primary colors (different from the primary colors of red, yellow, and blue), meaning that they contain molecules that absorb light—making the pigment appear a certain color depending on which wavelengths are absorbed.
But many examples of beautifully vibrant colors in nature are instead structural colors, which differ from primary colors in that structural colors use nanostructures to scatter light—often making these colors appear iridescent because they reflect light differently depending on the viewing angle.
UV radiation breaks down molecules and their bonds in primary colors. But nanostructures keep their shape in the sun, so structural colors don’t fade over time.
So scientists and manufacturers have long tried to fabricate synthetic structural colors for paints, pigments, and dyes.
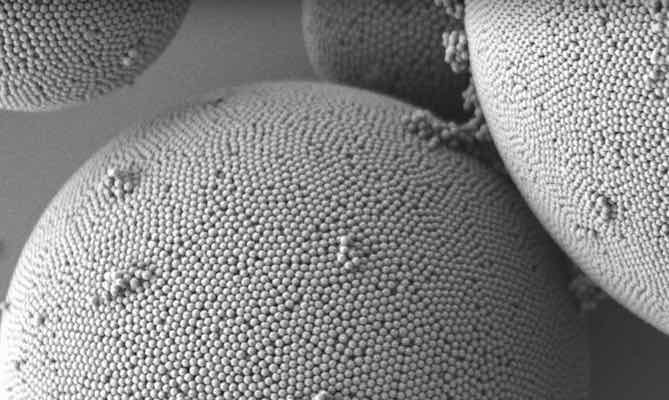
Now, researchers report a simple method to manufacture biocompatible structural colors using only melanin and silica. The silica shell provides a buffer layer of tunable thickness that allows customization of the particular color, offering the potential to fabricate a new breed of long-lasting pigments that don’t fade.
To create the new class of structural colors, the scientists started with synthetic melanin particles. Melanin is a natural molecule that gives color to our skin and hair. It’s also the molecule responsible for shielding our skin—or failing to do so—from the sun’s harmful radiation.
The team then coated the synthetic melanin particles in a layer of silica. Silica, or silicon dioxide, is a widely used material in a variety of industries, including food, pharmaceutical, and construction (an estimated 95% of produced silica goes into construction, including the production of Portland cement). Plus, silica is the primary ingredient used to produce many kinds of glass.
A water–oil emulsion technique concentrated the silica-encased melanin nanoparticles into aggregated supraballs, which the scientists isolated into a powdered pigment by simply dehydrating the solution.

Credit: Science Magazine; YouTube
The method is simple, economical, and scalable, offering a potential new pathway to manufacture a wide array of structural colors. Plus melanin and silica are both biocompatible, so such structural colors could be used to pigment foods, cosmetics, pharmaceuticals, and even tattoo inks.
Watch the short video below from Science Magazine to learn more.
The open-access paper, published in Science Advances, is “Bioinspired bright noniridescent photonic melanin supraballs” (DOI: 10.1126/sciadv.1701151).

Credit: Science Magazine; YouTube
Did you find this article interesting? Subscribe to the Ceramic Tech Today newsletter to continue to read more articles about the latest news in the ceramic and glass industry! Visit this link to get started.
Author
April Gocha
CTT Categories
- Biomaterials & Medical
- Manufacturing
- Material Innovations
- Nanomaterials
- Weekly Column: “Other materials”
Spotlight Categories
- Member Highlights

