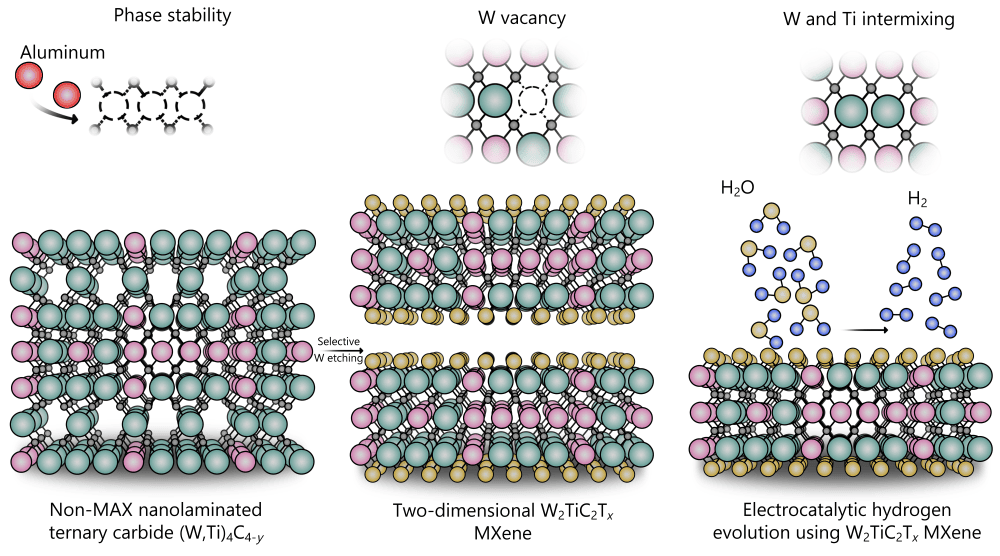
[Image above] Batteries undergoing testing. Credit: UCL Mathematical an Physical Sciences; Flickr CC BY 2.0
Sometimes, two is just better than one.
Research from the University of Illinois at Chicago shows that magnesium ions—with two positive charges—can replace single-charge lithium ions in future batteries.
“Because magnesium is an ion that carries two positive charges, every time we introduce a magnesium ion in the structure of the battery material we can move twice as many electrons,” says Jordi Cabana, UIC chemistry professor and principal investigator of the research, in a university press release. “We hope that this work will open a credible design path for a new class of high-voltage, high-energy batteries.”
Twice as many electrons mean twice as much battery juice with the same amount of material, which is important because batteries are not perfect.
“In our case, we want to maximize the number of electrons moved per ion, because ions distort the structure of the electrode material when they go in or leave. The more the structure is distorted, the greater the energy cost of moving the ions back, the harder it becomes to recharge the battery.”
The work shows that Mg2+ ions reversibly intercalate into a spinel-type manganese oxide, with the ions occupying tetrahedral sites of the structure.
Although they haven’t yet built a fully functional battery, the UIC scientists proved the principle that will make future batteries possible.
“Like a parking garage, there are only so many spaces for the cars,” Cabana said. “But you can put a car in each space with more people inside without distorting the structure.”
More charge means more power from a battery with the same amount of material, opening the door to batteries that can last longer or are lighter and smaller, both important considerations for longer-lasting electric cars, devices, and tech.
The paper, published in Advanced Materials, is “Direct observation of reversible magnesium ion intercalation into a spinel oxide host” (DOI: 10.1002/adma.201500083).
The UIC team isn’t the only one working towards better batteries—scientists at Kansas State University haven’t yet given up on lithium-ions. Their work shows that thin sandwiches of molybdenum disulfide sheets wrapped in ceramic silicon carbonitride show promise as battery electrodes. The sheets store twice the amount of lithium (akin to the double charge of magnesium) than previously reported bulk molybdenum disulfide. And, the electrodes don’t have the problem of capacity fading. The paper, published in Scientific Reports, is “Polymer-derived ceramic functionalized MoS2 composite paper as a stable lithium-ion battery electrode” (DOI: 10.1038/srep09792).
And just like electrodes coated in ceramic, glass coatings can improve batteries, too, according to research from scientists at the University of California, Riverside. To realize the potential of lithium–sulfur batteries—which can potentially produce 10 times as much energy as conventional batteries but also have stability problems—the team fabricated nanosized sulfur particles and coated them in glass, which traps problematic polysulfide compounds that form in these batteries. The paper, published in Nanoscale, is “SiO2-coated sulfur particles with mildly reduced graphene oxide as a cathode material for lithium–sulfur batteries” (DOI: 10.1039/C4NR07663J).
And then there are advances to improve zinc–air batteries, iron–fluoride batteries, and enhanced conductivity in ceramic composites like gadolinium-doped ceria.
And, if you’ve ever dreamt of watching battery cycles under the microscope, you’re in luck—researchers at Pacific Northwest National Lab have now visualized the formation of lithium dendrites, which lead to the demise of rechargeable lithium batteries.
What do you think is the most promising direction for tomorrow’s better batteries?
Author
April Gocha
CTT Categories
- Electronics
- Energy
- Material Innovations