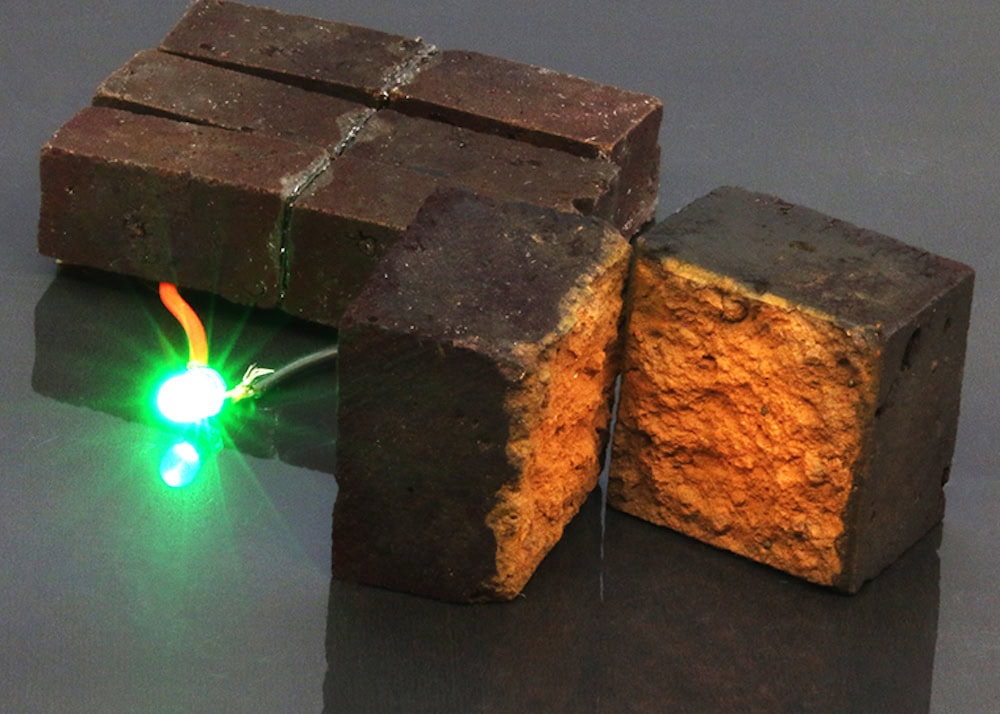
[Image above] Example of polymer-coated bricks that store energy like a battery. When connected in series, the bricks serve as a supercapacitor module capable of powering a green light-emitting diode. Credit: Wang et al., Nature Communications (CC BY 4.0)
When I hear the word “brick,” the first thing I often think of is “The Three Little Pigs.” Growing up, that fable was the basis of my knowledge about bricks—that they are small, red, rectangular clay units used to build sturdy, long-lasting walls.
Ever since I started working at ACerS, though, I realized bricks are much more than the red clay units featured in the little pigs’ tale. Bricks come in numerous colors and materials, with new compositions developed every year.
Beyond the different materials used, what I find most interesting are applications for bricks beyond architecture. Of course, refractory applications are an obvious one, but in this case I’m referring to unusual applications that occasionally appear in academic journals.
I wrote about one such application this April, a case in which researchers used bricks as “gamma ray cameras” to detect the previous presence of radioactive materials. And today, I feature another application—bricks used as energy storage units to hold electricity.
These brick batteries were created by researchers at Washington University in St. Louis. And to understand how they turned bricks into batteries, we first need to talk about an emerging field of materials science called organic electronics.
Organic electronics: A complement to classical electronics
Organic electronics is an emerging field of electronics based on organic materials, such as some polymers and organic molecules.
Compared to classical electronics, which are based on inorganic materials, organic electronics are more flexible, cover larger areas, and cost less to produce. However, organic electronics have lower energy density and reduced performance next to inorganics, so it is best to view the technologies as complementary rather than competing.
One polymer that is being researched extensively for organic electronics is poly(3,4-ethylenedioxythiophene), or PEDOT. PEDOT possesses excellent chemical and physical stability as well as high electrical conductivity.
PEDOT can be synthesized in several ways, but vapor-phase polymerization is a particularly promising strategy. This process, which uses an oxidizing agent in vapor form to cause molecules called monomers to bond together to form polymers, results in conformal coatings of low electrical resistance in a single step.
Usually, ferric (Fe3+) ions are used as the oxidizing agent, and they are introduced into the process in the form of ferric-ion-containing salts. However, ferric-ion-containing salts are corrosive, can be quite expensive, and are chemically unstable because they undergo hydrolysis over time, which challenges the reproducibility of experiments.
The PEDOT vapor-phase polymerization process could be less expensive and more sustainable if an alternative source of ferric ions is used—and that’s what the Washington researchers identified in their research.
Producing PEDOT from hematite
In nature, there are three main oxides of iron: hematite (Fe2O3), magnetite (Fe3O4), and wüstite (FeO).
Hematite is the most abundant of the three oxides. Its use as a pigment dates back thousands of years, and in modern times it is used extensively to produce iron and steel products.
As you can probably guess, hematite contains ferric ions. And compared to ferric-ion-containing salts, hematite is safe to handle and affordable.
In 2019, the Washington researchers published a study exploring how they might separate ferric ions from hematite for use in the PEDOT polymerization process.
They used hydrochloric acid vapor to dissolve rust, a hydrated form of hematite (Fe2O3·nH2O). The reaction freed aquated ferric ions to react with monomer vapor and initiate the polymerization of PEDOT into freestanding, nanofibrillar films.
Upon testing, the PEDOT films were found to have high conductivity and high electrochemical stability, thus showing “Rust-based vapor-phase polymerization is a novel approach for producing high-performing conducting polymers,” they write.
Following this study, the researchers began to consider ways to maximize the potential of this synthesis method. And that is where bricks come in.
Conventional red bricks get their red coloring from hematite, meaning they contain the ferric ions necessary to initiate vaper-phase polymerization.
Compared to the rusted steel sheet used in the original study, “A fired brick’s open microstructure, mechanical robustness and ~8 wt% α-Fe2O3 content afford an ideal substrate for developing electrochemical PEDOT electrodes and stationary supercapacitors that readily stack into modules,” the researchers write in a new paper.
Using the synthesis process developed in 2019, they dissolved the hematite contained in bricks to enable a reaction that coated the bricks in a PEDOT film. They then showed how these PEDOT-coated bricks could be used as electrodes in a symmetric supercapacitor.
In a Washington press release, assistant professor of chemistry Julio D’Arcy explains the potential these bricks have to transform the built environment.
“PEDOT-coated bricks are ideal building blocks that can provide power to emergency lighting,” he says. “We envision that this could be a reality when you connect our bricks with solar cells—this could take 50 bricks in close proximity to the load. These 50 bricks would enable powering emergency lighting for five hours.”
In addition, “a brick wall serving as a supercapacitor can be recharged hundreds of thousands of times within an hour. If you connect a couple of bricks, microelectronics sensors would be easily powered,” he adds.
I am excited to see this technology developed further—if it succeeds, “power bricks” will be a literal term in the future!
The 2019 paper, published in ACS Applied Energy Materials, is “Converting rust to PEDOT nanofibers for supercapacitors” (DOI: 10.1021/acsaem.9b00244).
The 2020 open-access paper, published in Nature Communications, is “Energy storing bricks for stationary PEDOT supercapacitors” (DOI: 10.1038/s41467-020-17708-1).
Author
Lisa McDonald
CTT Categories
- Energy
- Material Innovations


