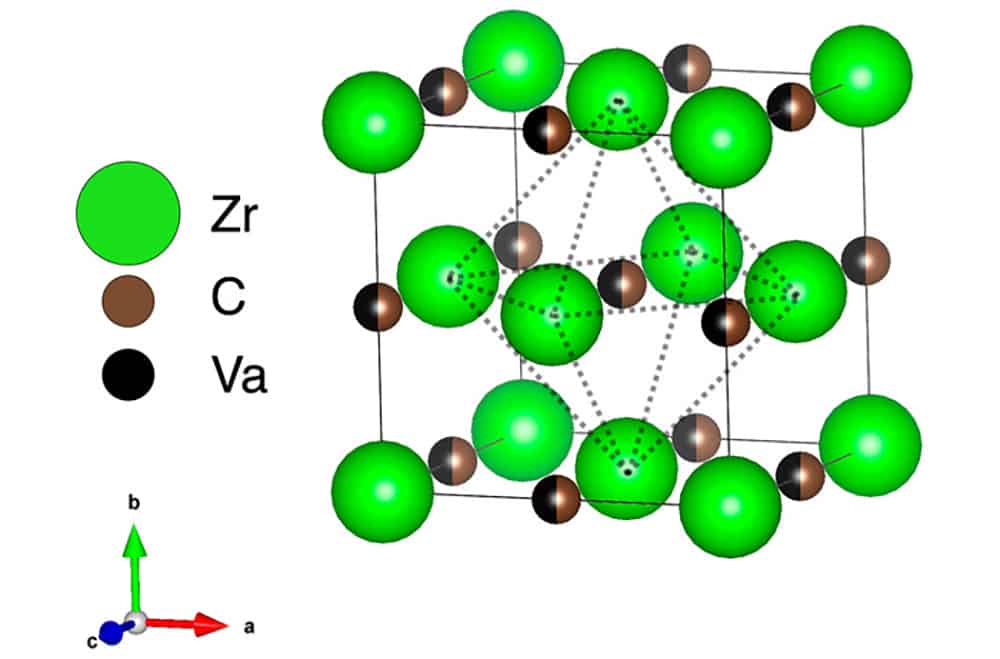
[Image above] Unit cell of a ZrCx lattice, where partial occupancy of the octahedral interstitial sublattice by carbon atoms and vacancies is indicated with mixed-color atoms. Credit: Davey and Chen, International Journal of Ceramic Engineering & Science (CC BY 4.0)
The only time one-size-fits-all clothing lives up to its name is in “The Sisterhood of the Traveling Pants.” Yet while that story achieved this feat through magical realism, there are some materials that can fulfill numerous tasks with ease thanks to the ability to form in multiple stable compositions and structures.
Zirconium carbide is one material with this ability. More than a century and a half of extensive study has shown this ultrahigh-temperature ceramic forms in a wide range of stable compositions with varying properties.
The composition variation in zirconium carbide is facilitated by varying numbers of carbon vacancies in the material’s structure. Much research has established that the number of these vacancies significantly affects the thermodynamic and thermophysical properties of the material. However, while one may naturally expect that the arrangement of vacancies affects the properties as well, little is known about these effects due to the challenging nature of relevant experimental studies.
Researchers have made significant strides in recent years toward understanding the effects of vacancy arrangement through careful theoretical considerations. For those interested in these effects, a new open-access review paper offers a comprehensive and thoughtful look at this topic.
ACerS member Theresa (Tessa) Davey, assistant professor at Tohoku University in Japan, wrote the paper along with fellow Tohoku professor Ying Chen. Davey’s research has focused extensively on point defects in zirconium carbide, and she previously developed a method to directly incorporate certain defect-related properties, such as formation energies, in a CALPHAD model.
In addition to summarizing the existing experimental and theoretical studies on vacancy arrangement in zirconium carbide, Davey and Chen also highlight areas where more studies are needed for further understanding. Below are some key points from the 24-page review.
Mechanism of vacancy ordering
In early studies on zirconium carbide, much attention was given to the structure of unit cells within the material. These cells consist of a fully occupied face-centered cubic lattice containing zirconium atoms and a sublattice formed by octahedral interstitial sites containing a mixture of carbon atoms and vacancies.
The focus on short-range ordering led early studies to report zirconium carbide as a solid solution, or a material family that has a range of compositions and a single crystal structure. However, in 1967, Goretzki performed neutron powder diffraction on titanium-carbon and zirconium-carbon alloys of several compositions, and he observed the existence of a separate ordered zirconium carbide structural phase at low carbon concentrations.
Davey and Chen write that long-range ordering can explain this observation. “To form an ordered phase, the carbon atoms and the vacant sites of the octahedral interstitial lattice are arranged with long-range ordering, generating a superstructure where the repeating units may be larger than the parent rock salt unit cell or may have significantly different symmetry,” they write.
Following Goretzki’s study, several groups tried to clarify zirconium carbide’s structure and revealed further ordered phases in the process. In 1977, de Novion and Maurice reviewed the information related to short- and long-range ordering of vacancies in transition metal carbides. They determined that long-range ordering of vacancies was driven by short-range ordering, which in this case tended to avoid second nearest-neighbor vacancy pairs. Future theoretical works validated this early view.
Following Novion and Maurice’s review in 1977, only a few more experimental studies on the structure of zirconium carbide were published in that decade before interest dropped off. No new experimental studies appeared in the literature until the 2010s, when interest in this material revived due to increased global initiatives toward developing nuclear fuel coatings for use in high-temperature gas-cooled reactors and hypersonic aviation. Theoretical studies began appearing in the literature as well, thanks to increasingly available, efficient, and accurate supercomputing resources.
The challenge with experimental studies
While theoretical studies have predicted several stable ordered phases, experimental observation of these phases remains limited. Davey and Chen state there are several possible explanations for this scarcity.
Hypothesis one: Synthesis temperatures are too high
Davey and Chen explain that during synthesis, zirconium carbide may be heated to about 2,000°C. However, at about 1,473–1,673°C, zirconium carbide experiences an order–disorder phase transition. Synthesizing the material above this transition temperature could lead to a disordered solid solution phase being frozen-in during quenching or cooling, rather than the ordered phases.
Synthesizing zirconium carbide at lower temperatures could address this issue. In 2019, Zhou et al. successfully synthesized zirconium carbide at 1,300°C.
Hypothesis two: Significant amounts of impurities
Significant amounts of impurities, such as nitrogen and oxygen, commonly occur in experimentally synthesized zirconium carbide and may affect vacancy ordering. “However, precise characterization of impurity concentration in zirconium carbide is notoriously challenging, which can mean that impurity concentrations are not accurately reported,” Davey and Chen write.
Further modeling studies such as the one conducted by Davey et al. could help identify processing parameters that mitigate the chance of impurities.
Hypothesis three: Composition too close to stoichiometric zirconium carbide
Carbon (or vacancy) diffusion in zirconium carbide is influenced by the vacancy composition—carbon diffusivity increases with higher vacancy concentration, and vice versa. “This offers a further possible explanation for the limited observances of other ordered phases closer to stoichiometric zirconium carbide [where vacancy concentration is lower],” Davey and Chen write.
Hypothesis four: Ordered phases are affecting each other
Currently, knowledge of all possible ordered phases is extremely limited, but it is theoretically feasible that a nonstoichiometric ordered phase may destabilize other predicted phases and prevent them from forming. “This, once again, offers a potential explanation for why Zr2C is the only consistently observed superstructural phase,” Davey and Chen write.
Next steps
Davey and Chen conclude the review by noting that experimental and theoretical studies to date confirm that vacancy arrangement as well as vacancy concentration significantly affect the thermophysical properties of zirconium carbide. However, the elastic and mechanical properties show conflicting results among different studies and thus require further work for clarification.
They suggest a few directions future research could take, including
- Experimentally confirm predicted stable structures: Recent theoretical work provides threshold values for temperature and impurity content that could assist in this area.
- Clarify the cause of variations in thermophysical properties: Some researchers have suggested these variations may be understood by considering the arrangements of vacancies and their pair cluster, although further work is needed to validate this hypothesis across all properties.
- Further explore short-range ordering behavior at high temperatures: The strong energetic preference for certain pair configurations in short-range ordering could explain why particular stoichiometries have such high melting points if the preference persists at high temperatures.
The open-access paper, published in International Journal of Ceramic Engineering & Science, is “Vacancy ordering in substoichiometric zirconium carbide: A review” (DOI: 10.1002/ces2.10126).
Author
Lisa McDonald
CTT Categories
- Basic Science