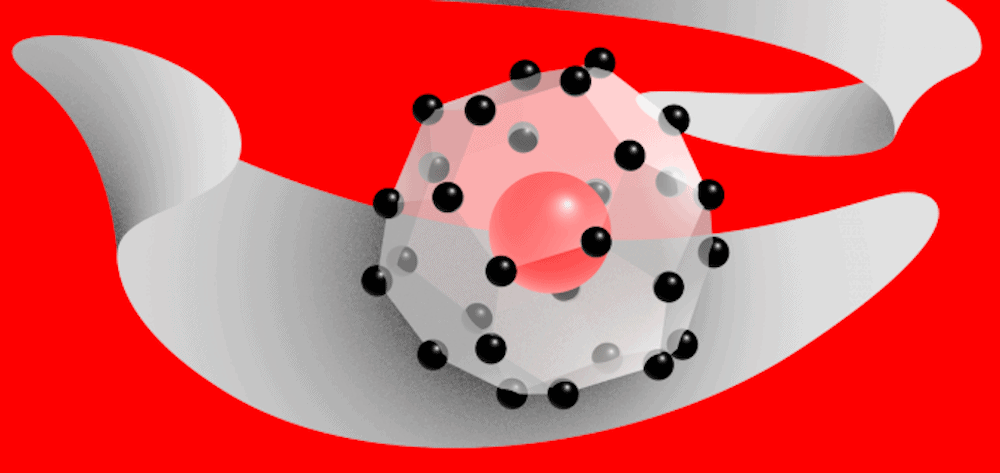
[Image above] Cerium superhydride, a “forbidden” compound, is possible under high pressure—but a pressure much lower than what is needed for other superconductors of the same type. Credit: @tsarcyanide, Moscow Institute of Physics and Technology
As we move into the last quarter of the year, the International Year of the Periodic Table nears its end. But there are still two and a half months before the International Year of Plant Health reaches our door, which gives us time to look at another cool discovery in the study of atomic structure.
In May, we discussed the discovery of oxygen-11, the lightest-ever isotope of oxygen to date. Today, we look at a molecule on the other end of the spectrum—instead of being super light, it packs more atoms than classical chemistry allows.
Metallic hydrogen or hydrides—which way to superconductivity?
“Superconductivity” is a buzzword in almost every science corner of the internet. It refers to a phenomenon whereby a charge moves through a material without resistance. Traditionally, achieving superconductivity required cooling a material to extremely cold temperatures (near absolute zero). For practical applications, however, superconductivity would need to be achieved closer to room temperature.
In the past few decades, scientists have expressed interest in the metallization of hydrogen under high pressure as a way to achieve high-temperature superconductivity. The possibility of a metallic modification of hydrogen is an old idea (see this article from 1935), but achieving metallization is not simple.
Hydrogen is expected to become metallic under high pressure above 400 GPa, a pressure at which many diamond anvil cell (DAC) experiments experience diamond failure. Additionally, verifying superconductivity is complicated by the fact DAC that experiments often lack a reliable probe with which to test the tiny sample volumes at such high pressures.
In contrast to metallic hydrogen, hydrogen-rich hydrides offer another possible way to achieve high-temperature superconductivity at potentially much lower pressure.
“Hydrogen-rich superhydrides are believed to be very promising high-Tc superconductors,” researchers of a new Nature Communications open-access paper begin their abstract. The researchers come from the United States, Russia, and China, and they explain the reason hydrides are so promising is because they may satisfy all conditions necessary for superconductivity: high phonon frequency, strong electron–phonon coupling, and high density of states at the Fermi level.
Superconductivity in hydrides
Already scientists have reaped some impressive results concerning superconductivity in hydrides, for example, the predicted and experimental confirmation of superconductivity in H3S at a record high Tc of 203 K (-70.15°C) under pressure of 150 GPa.
Recent theoretical predictions report several systems with unusually high hydrogen content (>H5) may exhibit significantly high Tc as well (examples here and here). However, some experimental studies on a few of these superhydrides required relatively high pressures to stabilize the molecule, for example, 170 GPa in LaH10.
A 2017 paper predicted hydrogen-rich cerium hydride (CeH9) may become stable at a relatively low pressure of 100 GPa. So the researchers of the recent paper chose to study the Ce–H system to search for superconductivity, with the hope the Tc value would be higher than the predicted value below 56 K (-217°C).
Creating a low-pressure superconductor
For their study, the researchers loaded a cerium sample and hydrogen gas into the sample chamber of a DAC and ranged pressure from 9 GPa to 100 GPa. At strategic points, they heated the sample with a pulsed laser to encourage transition of CeHx into different phases.
The researchers successfully synthesized four stable phases of CeHx (x = 2, 2.5, 3, and 9). The first three phases were known; CeH9 had only been predicted until this point.
Both the high pressure and pulsed laser heating were necessary to achieve CeH9, the synthesis of which was done by Nilesh Salke (Center for High Pressure Science and Technology Advanced Research, China) and Jung-Fu Lin (The University of Texas at Austin, U.S.).
“[Creating CeH9] can happen only at conditions where CeH9 is stable, i.e., at sufficiently high pressure, and requires excess of hydrogen in the system (to react with CeH3 and lead to CeH9),” Artem R. Oganov, coauthor and professor at the Skolkovo Institute of Science and Technology and Moscow Institute of Physics and Technology (MIPT), explains in an email. “Such a chemical reaction has an activation barrier, to overcome which one needs to have sufficiently high temperature [the pulsed laser heating].”
The researchers found the DAC results agreed with their theoretical simulations—theory predicted CeH9 became stable at pressures above 78 GPa; in experiment, CeH9 was stable when synthesized between 80–100 GPa. Specifically, CeH9 was stable when in the P63/mmc structure.

Pressure temperature path for synthesis and stability of various Ce–H phases. a Starting at 9 GPa, cerium reacts with hydrogen to form 3¯ -CeH2, which remains stable up to 33 GPa. b At 33 GPa with laser heating, 3¯ -CeH2 in H2 medium reacts to form – 3¯ -CeH3, which remains stable up to 80 GPa. c Laser heating of – 3¯ -CeH3 in H2 medium at 80–100 GPa results in the occurrence of P63/mmc-CeH9 superhydride. d After complete decompression, – 3¯ -CeH3 and 41 -Ce2H5 are recovered at ambient conditions. Credit: Salke et al., Nature Communications (CC BY 4.0)
Superconductivity in CeH9
In hydrides, interaction with metal atoms causes the hydrogen to precompress; as such, the hydrogen sublattice in hydrides might be expected to mirror atomic metallic hydrogen (and thus have higher potential for superconductivity). The researchers found CeH9 had a smaller H–H bond length than other reported hydrides and is second only to the H–H distance for atomic metallic hydrogen at 500 GPa.
Despite this structural similarity to monatomic metallic hydrogen, the predicted Tc for CeH9 [105–117 K (below -150°C)], though higher than the earlier predicted value of 56 K, is not as high as that of metallic hydrogen nor LaH10 [250 K (-23°C) at 170 GPa]. “The shortest H-H distance is not the only thing that matters for high Tc; the electronic structure of the metal atom plays a crucial role,” the researchers explain in the paper.
However, “this material [CeH9] is remarkable in that it is stable at a pressure of 1 million atmospheres—less than what the previously synthesized sulfur and lanthanum superhydrides require,” Ivan Kruglov, coauthor and researcher at MIPT and Dukhov Research Institute of Automatics, says in an MIPT press release.
Currently, Oganov says they are now working on the yttrium-hydrogen system because “This system was predicted to have room-temperature superconductor YH10.”
The open-access paper, published in Nature Communications, is “Synthesis of clathrate cerium superhydride CeH9 at 80–100 GPa with atomic hydrogen sublattice” (DOI: 10.1038/s41467-019-12326-y).
Author
Lisa McDonald
CTT Categories
- Basic Science