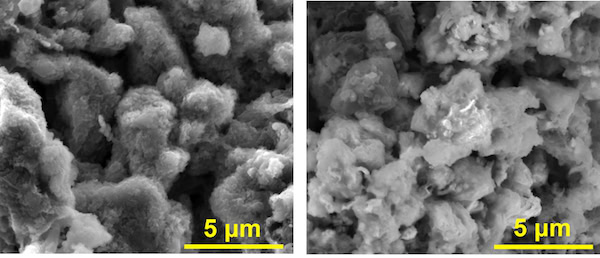
[Image above] Scanning electron microscope images show an anode of asphalt, graphene nanoribbons, and lithium at left, and the same material without lithium at right. The material was developed at Rice University and shows promise for high-capacity lithium batteries that charge 20 times faster than commercial lithium-ion batteries. Credit: Tour Group; Rice University
There’s no shortage of research when it comes to making improvements to lithium batteries. Researchers continue to experiment on making them safer, cheaper, and faster-charging.
And scientists continue to experiment with incorporating various materials in batteries—from glass and ceramic materials, to diatoms and diamonds.
Now, another material that may prove to be worth its weight in faster charging times is “resurfacing” at Rice University. A team of researchers led by chemist James Tour has developed lithium battery anodes made of porous carbon derived from asphalt that have the capability of charging lithium metal batteries 10–20 times faster than current lithium-ion batteries already on the market.
Last year we reported on research Tour’s group had conducted using a material called Gilsonite, a type of asphalt that the scientists developed to remove impurities like greenhouse gases from natural gas.
For this research, the group created a battery anode by mixing untreated Gilsonite with conductive graphene nanoribbons and then coating it with lithium metal. The cathode was made of sulfurized carbon.
During testing, the batteries displayed a high power density of 1,322 watts per kg and high energy density of 943 watt-hours per kg, according to a Rice University press release.
“The capacity of these batteries is enormous, but what is equally remarkable is that we can bring them from zero charge to full charge in five minutes, rather than the typical two hours or more needed with other batteries,” Tour says in the release.
One problem that typically plagues lithium batteries (and challenges researchers) is the formation of dendrites over time, after a battery’s repeated charges and discharges. These dendrites grow inside the electrolyte, eventually short-circuiting the battery, causing spontaneous combustion and fires.
But Tour’s team solved this problem, too—apparently carbon is a dendrite killer. The Gilsonite they used in the battery anode was enough to prevent dendrite formation.
Although the research team’s breakthrough is notable for electronics such as laptops, smartphones, and other portable devices that use lithium batteries, the more significant application is in the next generation of electric vehicles.
“This technology could be just what manufacturers need for the next generation of electric cars,” Tour writes in an email. “Batteries that charge quickly without sacrificing storage capacity are going to go a long way toward making electric cars practical, especially for city dwellers, and the benefits to the environment are obvious.”
The paper, published in ACS Nano, is “Ultrafast charging high capacity asphalt–lithium metal batteries” (DOI: 10.1021/acsnano.7b05874).
Did you find this article interesting? Subscribe to the Ceramic Tech Today newsletter to continue to read more articles about the latest news in the ceramic and glass industry! Visit this link to get started.
Author
Faye Oney
CTT Categories
- Basic Science
- Electronics
- Energy
- Nanomaterials


